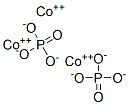The anhydrous Co3(PO4)2 is obtained when cobalt(II) salts are heated with phosphoric acid. The octahydrate Co3(PO4)2.8H2O is precipitated when aqueous solutions of cobalt(II) chloride and potassium hydrogen phosphate are mixed. As it is a brilliant lavender colour it is used in artists' paints and as a pigment in ceramics. The pyrophosphate Co2P2O7.8H2O is similarly precipitated using sodium pyrophosphate and a cobalt(II) solution.
Cobalt phosphate, Co3(PO4)2, is a commercial inorganic pigment known as cobalt violet. Thin films of this compound are water oxidation catalysts. The tetrahydrate CoPO4(H2O)4 precipitates as a solid upon mixing aqueous solutions of cobalt(II) and phosphate salts. Upon heating, the tetrahydrate converts to the anhydrous compound. The
anhydrous CoPO4 comprises discrete phosphate (PO32
4 ) anions that link Co21 centers. The cobalt ions occupy both
octahedral (six-coordinate) and pentacoordinate sites in a 1:2 ratio.