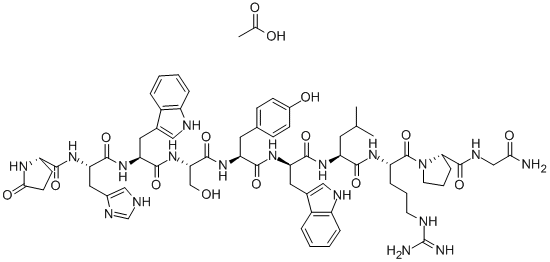
Triptorelin acetate (TA) is a GnRH agonist that was earlier used as a veterinary drug for a single fixed-time artificial insemination through the synchronised estrous cycle of weaned sows. The risk assessment was that the Food Safety Commission of Japan (FSCJ) concluded that TA is not likely to cause adverse effects on human health through dietary exposure. Because TA does not have genotoxicity relevant to human health and is degraded in the digestive tract, its oral bioavailability is low. TA is currently used for the treatment of central precocious puberty and assisted reproduction. Injectable TA alone or in combination with levonorgestrel intrauterine device is also used to treat endometrial atypical hyperplasia (EAH) . Combination therapy is more effective, improving patients' menstrual abnormalities, endometrial thickening and quality of life, and is safe.
Synthetic peptide agonist analog of LH-RH. Triptorelin acetate is a potent agonist of LH-releasing hormone, currently used in the treatment of prostatic cancer where therapy may be required over months or years. Antineoplastic (hormonal). Used in the treatment of endometriosis and infertility.
Synthetic peptide agonist analog of LH-RH. Triptoreline is a potent agonist of LH-releasing hormone, currently used in the treatment of prostatic cancer where therapy may be required over months or years. Antineoplastic (hormonal). Used in the treatment of endometriosis and infertility.
Triptorelin acetate has been reported to have a possible side effect of severe bone loss. The first report was of an 8-year-old girl with central precocious puberty who experienced anaphylactic shock after the first injection of decapeptide containing triptorelin acetate (D,L lactide coglycolide), dextran 70, and polysorbate 80. However, it is also possible that it was a reaction to the polysorbate. Therefore, doctors should be aware of immediate reactions to drugs containing polysorbates.