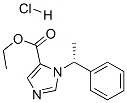
Hypnomidate,Janssen,W. Germany,1977
To a mixture of 1,115 parts dl-1-phenylethylamine and 950 parts
dimethylformamide are added successively 655 parts triethylamine and 1,130
parts ethyl chloroacetate. After the addition is complete, the whole is stirred
overnight. Then there are added 5,600 parts anhydrous ether and the whole
is filtered.
The filtrate is washed four times with water, dried and evaporated, yielding dl-
N-[(ethoxycarbonyl)methyl] -1-phenylethylamine. This residue is dissolved in 4,800 parts xylene while refluxing and to this solution are added 450 parts
formic acid. After boiling for a few hours, the mixture is cooled and washed
successively three times with a 20% solution of formic acid, water, sodium
hydrogen carbonate solution.
The organic layer is then dried, filtered and evaporated. The oily residue is
distilled in vacuo, yielding 1,600 parts dl-N-formyl-N-
[(ethoxycarbonyl)methyl]-1-phenylethylamine (boiling point 160°C to 170°C
at 0.8 mm pressure). 30 parts of a sodium dispersion, 50% in paraffin oil are
added to 450 parts tetrahydrofuran and the whole is slowly heated to a
temperature of 40°C, while stirring. While maintaining this temperature
(cooling on a water bath is necessary) there are added portionwise 30 parts
ethanol.
After the addition is complete, the whole is cooled on an ice bath and there is
added dropwise a solution of 144 parts dl-N-formyl-N-
[(ethoxycarbonyl)methyl]-1-phenylethylamine in 133 parts ethyl formate.
After the addition is complete, the mixture is stirred overnight at room
temperature.
Then there are added 160 parts ether. After stirring for 5 minutes the mixture
is poured into 1,500 parts water. The aqueous layer is separated, washed
twice with 80 parts diisopropyl ether and then there are added successively
114 parts concentrated hydrochloric acid and 90 parts potassium thiocyanate
in 200 parts water. The mixture is stirred for 24 hours, where upon an oil is
separated.
After the addition of 750 parts water, a crystalline product is precipitated. The
mixture is further stirred overnight. The solid is then filtered off and
recrystallized from a mixture of ethanol and water (1:1 by volume) to yield dl-
1-(phenylethyl)-2-mercapto-5-(ethoxycarbonyl)imidazole; its melting point is
129.8°C to 130.8°C.
To a stirred mixture of 140 parts nitric acid (d = 1.37), 1 part sodium nitrate
and 240 parts water are added portionwise 89 parts dl-1-(1-phenylethyl)-2-
mercapto-5-(ethoxycarbonyl)imidazole. After the addition is complete, the
whole is stirred for 2 hours at room temperature. The free base is liberated by
addition of solid sodium carbonate and the whole is extracted with 120 parts
anhydrous ether while heating. The aqueous layer is separated and extracted
twice with 80 parts anhydrous ether.
The combined extracts are dried over magnesium sulfate, filtered and to the
filtrate is added 2-propanol previously saturated with gaseous hydrogen
chloride. The precipitated salt is filtered off, dried for 2 days at 60°C, to yield
dl-1-(1-phenylethyl)-5-(ethoxycarbonyl)imidazole hydrochloride. It has a
melting point 142°C to 142.8°C.