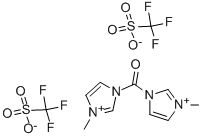
1,1′-Carbonylbis(3-methylimidazolium) Bis(trifluoromethanesulfonate) is a White to Off-White Solid, H δ (CDCl3) 8.86 (s, 2H), 7.44 (m, 2H), 7.16 (m, 2H), 4.00 ;(s, 6H); sol nitromethane, chloroform; insol ether; reacts with water.
An efficient reagent for aminoacylations. Used in the synthesis of chiral Pilocarpine analogs via a C-8 ketone intermediate.
As an efficient reagent for aminoacylations, 1,1'-Carbonylbis(3-MethyliMidazoliuM) Triflate can be used in the synthesis of chiral Pilocarpine analogs via a C-8 ketone intermediate.
Preparative Methods of 1,1′-Carbonylbis(3-methylimidazolium) Bis(trifluoromethanesulfonate): fresh methyl triflate is added to 1,1-carbonylbisimidazole in nitromethane at 10 °C.
Solvent is then removed in vacuo, or the reagent used in situ[1].
1,1′-Carbonylbis(3-methylimidazolium) Bis(trifluoromethanesulfonate) is usually prepared under anhydrous conditions and used immediately.
It can be stored in dry nitromethane solution or in solid state in absence of moisture. Preparation using aged
methyl triflate should be avoided, owing to possible contamination of the coupling reagent with triflic acid[1].
1. Saha, A. K.; Schultz, P.; Rapoport, H. JACS, 1989, 111, 4856.