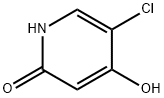
Compound 6 (8.0 g, 44 mmol) was used as a raw material, which was mixed with acetonitrile (100 mL), acetic acid (3 mL) and sodium iodide (13.2 g, 88 mmol), and the reaction was heated at 60°C for 8 hours. Upon completion of the reaction, the mixture was cooled to room temperature and poured into 10% sodium thiosulfate solution (200 mL) to precipitate a colorless solid. Purification by aqueous recrystallization afforded the target product 5-chloro-4-hydroxypyridin-2(1H)-one (Compound 2) in a yield of 5.6 g in 86%. The product characterization data were as follows: melting point 272-273 °C (literature value 273-274 °C); 1H NMR (400 MHz, DMSO-d6) δ 5.70 (s, 1H), 7.51 (s, 1H), 11.29 (br, 2H); 13C NMR (100 MHz, DMSO-d6) δ 163.5, 163.2, 134.6 , 105.6, 98.7; LRMS (ESI) m/z (%): 146 (100) [M(35Cl)+H]+, 148 (30) [M(37Cl)+H]+; HRMS (ESI) m/z calculated value of C5H535ClNO2: [M+H]+: 146.0003, measured value: 146.0012; C5H537ClNO2: [M+H]+: 147.9975, measured value: 147.9975.