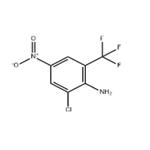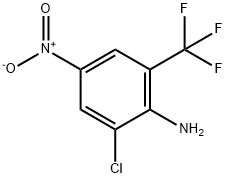
2-AMINO-3-CHLORO-5-NITROBENZOTRIFLUORIDE
- Product Name2-AMINO-3-CHLORO-5-NITROBENZOTRIFLUORIDE
- CAS400-67-9
- MFC7H4ClF3N2O2
- MW240.57
- EINECS225-202-8
- MOL File400-67-9.mol
Chemical Properties
| Melting point | 115-116 °C(Solv: acetic acid (64-19-7)) |
| Boiling point | 286.0±35.0 °C(Predicted) |
| Density | 1.614±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| pka | -3.90±0.46(Predicted) |
| CAS DataBase Reference | 400-67-9(CAS DataBase Reference) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 26-36/37/39 |
| Hazard Note | Irritant |
| HS Code | 2921490090 |