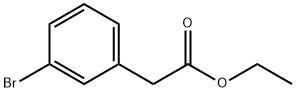
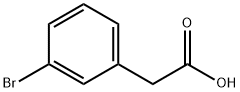
To a 250 mL round bottom flask was added 3-bromophenylacetic acid (5 g, 23.25 mmol, 1.00 equiv) and ethanol (80 mL). With stirring at 0 °C, thionyl chloride (5.6 g, 46.28 mmol, 2.00 eq.) was added slowly and dropwise. The reaction mixture was heated to reflux and kept for 2 hours. Upon completion of the reaction, the mixture was concentrated under reduced pressure to remove the solvent. Subsequently, the reaction was quenched by the addition of water. The aqueous phase was extracted with ethyl acetate (3 × 30 mL) and the organic layers were combined. The organic layer was washed with saturated saline (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. 5.5 g (97% yield) of ethyl 2-(3-bromophenyl)acetate was finally obtained as a colorless oil.
[1] Journal of Medicinal Chemistry, 2000, vol. 43, # 8, p. 1508 - 1518
[2] Patent: US2013/190290, 2013, A1. Location in patent: Paragraph 1328; 1329; 1330
[3] Patent: WO2015/130957, 2015, A1. Location in patent: Page/Page column 77
[4] Patent: WO2010/127440, 2010, A1. Location in patent: Page/Page column 39-40
[5] Patent: WO2010/127448, 2010, A1. Location in patent: Page/Page column 26-27