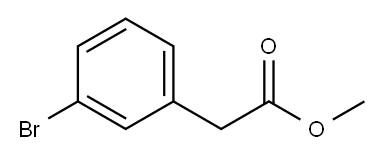
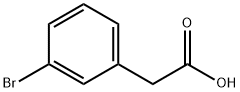
General procedure for the synthesis of methyl (3-bromophenyl)acetate from methanol and 3-bromophenylacetic acid: acetyl chloride (0.08 mL, 1.2 mmol, 0.5 eq.) and 3-bromophenylacetic acid (0.5 g, 2.3 mmol, 1.0 eq.) were added to methanol (10 mL), and the reaction mixture was refluxed for 2 hours. Upon completion of the reaction, the consumption of starting material was confirmed by TLC monitoring. The reaction mixture was concentrated and extracted with dichloromethane (DCM). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford methyl (3-bromophenyl)acetate as a colorless oil (0.525 g, 98.7% yield). The product was analyzed by LC-MS (ES), m/z = 229.0, 231.0 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 3.61 (s, 3H), 3.70 (s, 2H), 7.26-7.30 (m, 2H), 7.43-7.46 (m, 1H), 7.48 (s, 1H).