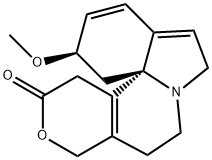
[2R-(2R*,13bS*)]-2,6,8,9,10,13-Hexahydro-2-methoxy-1H,12H-benzo[i]pyrano[3,4-g]indolizin-12-one,
ChEBI: An organic heterotetracyclic indole alkaloid isolated from the seeds and other parts of Erythrina species. It differs from the alpha isomer in having the double bond of the dihydropyranone ring located beta, <greek gamma- to the lactone carbonyl group instead of alpha,beta-.
Purify it like the -isomer but recrystallise it from EtOH. The free base is unstable in air and light, but the hydrochloride is more stable and best stored as such. The methiodide has m 211o (prisms from EtOH). -Erythroidine hydrochloride can be obtained from the -isomeric salt as follows: The -hydrochloride (1.2g) in 10% aqueous NaOH (12mL) is refluxed for 3hours under N2, cooled in ice and conc HCl added to pH 2. After standing for 3hours, NaHCO3 is carefully added to pH 7, the solution is extracted with CHCl3 (5x), and the extracts are dried, filtered, and concentrated to give an oil which on seeding gives -erythroidine m 97-99o. When dissolved in EtOH and dry HCl gas is passed through the solution, the pure -erythroidine hydrochloride crystallises out with m 230.5-231.5o, [ ] D 25 +10o (c 0.5, H2O). It is a muscle relaxant with a curarelikeaction and is more active than the -isomer. [Koniuszy & Folkers J Am Chem Soc 72 519 1950, Boekelheide et al. J Am Chem Soc 75 2550 1953, Boekelheide & Morrison J Am Chem Soc 80 3905 1958, Wenzinger & Boekelheide J Org Chem 29 1307 1964, Berger & Schwartz J Pharmacol Exp Therap 9 3 362 1948, Beilstein 4 IV 3568.]