150.0 g (1 mol) of p-tert-butylphenol are dissolved in 1200 ml of anhydrous
ethyl ether and the thus obtained solution is added to a 10% solution of
ethylmagnesium bromide in 1000 ml of anhydrous ether, at room
temperature, under stirring.
After having evaporated the ether, 1000 ml of anhydrous benzene are added and the mixture is distilled, at normal pressure, until its volume is about 2/3
of the original volume. After cooling at room temperature, 1 mol of 1-methyl-
5-nitroimidazolyl-2-carboxyaldehyde in 1000 ml of anhydrous benzene is
added.
The whole is boiled for 1 h, is cooled to about 10°C and is added with 8% HCl
to adjust the pH to 7. Benzene layer is dried and concentrated up to 2/3 of
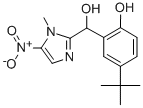
the volume. The 2-(1-methyl-5-nitro)imidazolyl-1-(2-hydroxy-5-tertbutylphenylcarbinol
is allowed to crystallize, melting point 158°-160°C; yield
0-60%.