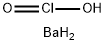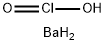Barium chlorite, Ba(ClO2)2, is a solid with the molecular
weight of 204.779 g/mol. Its density is 3.61 g/cm3.
Its CAS number is 14674-74-9. Its melting point is
727°C and it has a boiling point of 1640°C. Barium chlorite
can be prepared by reaction of chlorous acid with
barium carbonate:
BaCO3 (s)+ 2HClO2 (aq)→Ba(ClO2)2·7H2O
It is a white granular solid and if formed in solution,
crystallizes as the heptahydrate. Like its strontium
homologue, barium chlorite is a strong
oxidizing agent. It accelerates the burning of combustible materials, ignites on contact with potassium thiocyanate
and reaction with chlorine yields explosive
chlorine dioxide gas. Reaction with ammonia
produces ammonium chlorite, which is shock sensitive.
If mixed with finely divided metallic or organic
substances, the combination is highly flammable and
may be ignited by friction.