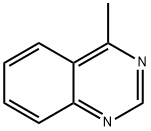
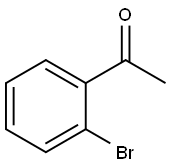
GENERAL METHODS: 2'-Bromoacetophenone (1a) (100.0 mg, 0.5 mmol), benzaldehyde (2a) (58.0 mg, 0.55 mmol), CuCl (5.0 mg, 0.05 mmol), 25% ammonia (0.5 mL), and NMP (0.5 mL) were sequentially added to a 25 mL thick-walled Pyrex screw-capped tube, which was sealed under an atmosphere of air sealed under air atmosphere. The reaction mixture was placed in an oil bath at 80°C and stirred for 12 hours. After the reaction was completed, it was cooled to room temperature and the crude product was extracted with EtOAc three times (3.0 mL each time). The organic phases were combined and concentrated under reduced pressure to remove the solvent. The residue was dissolved with CH2Cl2 (4.0 mL) and n-docosane (62.1 mg, 0.2 mmol) was added as an internal standard for GC analysis. After analyzing the reaction mixture by GC and GC-MS, the volatile solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography [eluent: petroleum ether, followed by a mixed solvent of petroleum ether/ethyl acetate (100:1 to 50:1)] to afford 4-methyl-2-phenylquinazoline (3a) (69.6 mg, 0.32 mmol, 63% yield) as a white solid.GC analysis showed that the GC yield of 3a was 65%.