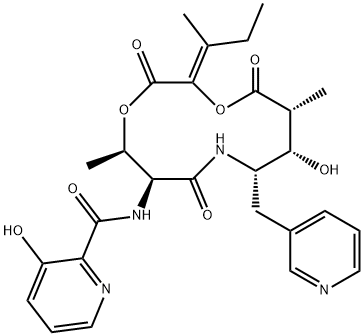
Pyridomycin is a structurally unusual antimycobacterial cyclodepsipeptide whose composition includes two rare moieties: 3-(3-pyridyl)-L-alanine and 2-hydroxy-3-methylpent-2-enoic acid. It inhibits NADH-dependent enoyl (Acyl-Carrier-Protein) reductase InhA, preventing mycolic acid synthesis in M. tuberculosis, including isoniazid-resistant strains (MICs = 0.31-0.63 μg/ml).
Pyridomycin is a potent antibiotic active against mycobacteria.
Pyridomycin is a potent antibiotic active against mycobacteria and some Gram negative bacteria, originally isolated from Streptomyces abidoflavus by Umezawa group at the NIH Japan in 1953, and since isolated from different species and published under several names. The unusual 12-membered macrocyclic depsipeptide comprises three unique sub-units incorporating two substituted pyridines. Pyridomycin is thought to target NADH-dependent enoyl (acyl-carrier-protein) reductase InhA. Recent reports of activity against isoniazid-resistant mycobacteria has seen pyridomycin identified as a potential lead for new generation antibiotics.