Benezrial,Houde,France,1978
2N sodium hydroxide solution (5 ml) is added to a stirred suspension of S�methylisothiosemicarbazide hydroiodide (2.33 g) and hydroxylamine
hydrochloride (0.70 g) in water (6 ml) and stirred for 48 hours. The solution is
evaporated in vacuo to provide 1-amino-3-hydroxyguanidine. One-third of the
residue is dissolved in 16 ml of ethanol and 2,6-dichlorobenzaldehyde (0.6 g)
is added to this solution. The reaction mixture is then stirred for 48 hours.
The solution is then evaporated in vacuo and the residue dissolved in ether
(30 ml) and in hydrochloric acid (30 ml). The aqueous phase is rendered
alkaline with 2N sodium carbonate solution and extracted with ether. The
ether layer is dried with sodium sulfate and evaporated. The residue is
dissolved in ether and excess dry hydrogen chloride is passed into the
solution.
The resultant mixture is evaporated in vacuo and the residue triturated with
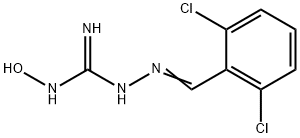
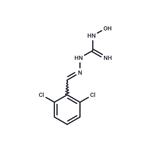
methylene chloride to afford a crude product. Recrystallization from ethanol�ether (1:3) provides 1-(2,6-dichlorobenzylideneamino)-3-hydroxyguanidine
hydrochloride; MP 173°C to 175°C. When the above process is carried out and
S-benzylisothiosemicarbazide hydroiodide is used in place of S-methylisothiosemicarbazide hydroiodide, the identical product is again
obtained.