39 g ethyl chloroformate are added dropwise to a mixture of 68.4 g 3- acetylamino-p-toluene sulphonic acid amide, 123 g potassium carbonate and 450 ml acetone for one hour while boiling under reflux. Refluxing is then continued for a further nine hours. The reaction mixture is cooled and mixed, while stirring, with a mixture of 450 ml water and 50 ml 2 N potassium hydroxide solution. Thereby two layers are formed. The upper layer, which consists of aqueous acetone, is separated. Acetone is distilled off in a vacuum. The pH-value of the resulting aqueous solution is adjusted to a pH of 8.8 by passing in gaseous carbon dioxide. Precipitated unchanged starting material is filtered off. The filtrate is rendered congo acid by the addition of dilute hydrochloric acid. The precipitated 3-acetylamino-p-toluene sulfnyl ethyl urethane is filtered off by suction, washed with water, and dried in a vacuum. The yield is 77%. The resulting compound melts at 183°-194°C.
54.3 g above prepared 3-acetylamino-p-toluene sulphonyl ethyl urethane are mixed with 37 ml dimethylformamide and 18 g cyclohexylamine. The resulting clear solution is heated at 70°C for 1.5 hours and at 110°C for 1.5 more
hours. After cooling, the reaction mixture is poured into 500 ml water while
stirring. The precipitated oily product crystallizes shortly. The crystals are
filtered off by suction, washed with water and dried in a vacuum. Yield of 3-
acetylamino-p-toluene sulphonyl cyclohexyl urea is 84%. MP: 174°C. The urea
is saponified without further purification by heating it in 90 ml 5 N potassium
hydroxide solution at 90°C for one hour. After dilution with 500 ml water the
resulting reaction mixture is rendered acid (pH 6.5) by the addition of dilute
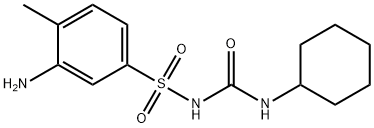
hydrochloric acid. Thereby, 1-(3-amino-p-tolylsulfonyl)-3-cyclohexylurea
separates in crystals, which are collected, washed with water, and dried. The
yield is 86%. After recrystallization from ethanol the compound has MP: 151°-
152°C.