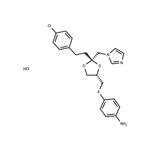
Usage And Synthesis
A stirred solution of 4-chlorophenethyl alcohol (131.0 g) and
triphenylphosphine (241.3 g) in dry THF (500 ml) at 0°C was treated portionwise
over 30 min with N-bromosuccinimide (163.75 g). The resulting black
solution was stirred overnight at room temperature, whereupon the THF was
evaporated and the residue stirred with ether. The solution was filtered and
the filtrate evaporated and treated with hexane. The stirred mixture was
filtered, evaporated, and the residue distilled under reduced pressure to give
100.0 g of 4-chlorophenethyl bromide as a colorless liquid, boiling point 85°C (3 mm Hg).
To a flame-dried flask containing magnesium turnings under ether was added
4-chlorophenethyl bromide in anhydrous ether at such a rate as to maintain a
gentle reflux. When the addition was complete, the mixture was heated under
reflux for an additional hour and then treated dropwise over 1 h with vinyl
bromide in ether maintaining a gentle reflux. The resulting mixture was stirred
overnight at room temperature and then poured onto ice-cold dilute sulfuric
acid. The product was extracted with ethyl acetate and the combined extracts
were washed with dilute aqueous potassium carbonate, dried MgSO4 and
evaporated. The resulting brown oil was distilled under reduced pressure to
give 4-(4-chlorophenyl)but-1-ene.
To a solution of 4-(4-chlorophenyl)but-1-ene in dichloromethane was added dropwise with stirring a mixture of 40% peracetic acid and sodium acetate. The resulting mixture was heated under reflux, cooled, and stirred with water. The dichloromethane layer was separated, washed with dilute aqueous potassium carbonate until neutral, water, and dried (MgSO4) and evaporated to give 4-(4-chlorophenyl)-1,2-epoxybutane as a colorless oil.
To a suspension of sodium hydride (50% dispersion in mineral oil) in dry DMF under nitrogen was added imidazole in dry DMF with stirring at such a rate as to keep the temperature below 65°C (ice bath). When the evolution of gas had ceased, 4-(4-chlorophenyl)-1,2-epoxybutane was added dropwise and the mixture stirred overnight at room temperature. The resulting brown solution was added water, extracted with ethyl acetate and the combined extracts were washed with water 3 times, dried (MgSO4) and evaporated to give an oil which crystallized. Washing the solid with ether and filtration give 1-(4-(4- chlorophenyl)-2-hydroxybutyl)imidazole.
A solution of oxalyl chloride (74.8 g) in dry dichloromethane (1350 ml) at below -70°C under a nitrogen atmosphere was treated with dry dimethylsulfoxide (91.5 ml) in methylene chloride (270 ml) dropwise over 15- 20 min while maintaining the temperature of the reaction mixture below - 50°C. After an additional 5 min a solution of (+/)-1-(4-(4-chlorophenyl)-2- hydroxybutyl)imidazole (128.3 g) in a mixture of dimethylsulfoxide (50 ml) and methylene chloride (200 ml) was added over a period of 20 min keeping the reaction mixture at temperatures below -65°C. After a further 15 min dry triethylamine (300 ml) was added rapidly and after 15 min the reaction mixture was allowed to warm to 0°C. Water (20 ml) was then added to the reaction mixture, the methylene chloride then removed by evaporation and the resulting slurry was then treated with water and filtered. The filter cake was washed well with ice water, cold ethyl acetate and then dried in air to yield 118.0 g of 4-(4-chlorophenyl)-1-(imidazol-1-yl)butan-2-one.
A mixture of 4-(4-chlorophenyl)-1-(imidazol-1-yl)butan-2-one (50.0 g), ptoluenesulfonic acid monohydrate (42.07 g) and glycerol (37.0 g) in toluene (200 ml) was heated under reflux, with stirring, through a Dean-Stark trap for 6 h. The two layers were allowed to separate and the hot toluene (upper layer) decanted and discarded. The lower layer was poured into 2 N sodium hydroxide (500 ml), the transfer completed by washing the flask with 1 N sodium hydroxide and methylene chloride, and the product extracted with methylene chloride (4x200 ml). The extracts were dried (MgSO4) evaporated and the residue recrystallized from toluene to give 61.4 g of (cis/trans)-2-(2- (4-chlorophenyl)ethyl)-2-(imidazol-1-yl)methyl-4-hydroxymethyl-1,3- dioxolane, melting point 96°-110°C.
(cis/trans)-2-[2-(4-Chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- hydroxymethyl-1,3-dioxolane (39.7 g) in pyridine (150 ml) at 0°C was treated drop-wise with stirring with methanesulfonyl chloride (10.6 ml) and the mixture stirred overnight. The resulting solid mass was stirred with ether (500 ml) to break up the solid, filtered and washed well with ether to give (cis/trans)-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane hydrochloride, melting point 107°- 110°C (recrystallized from dichloromethane/isopropanol).
The (cis/trans)-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane hydrochloride was basified with aqueous potassium carbonate solution, extracted with ethyl acetate (2x400 ml) and the extracts washed, dried (MgSO4) and evaporated. The resulting semicrystalline mass was chromatographed on silica gel (900.0 g) eluting with dichloromethane, aqueous ethyl acetate (2.2% water) to give 25.4 g of (+/-)- cis-2-(2-(4-chlorophenyl)ethyl)-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane, as a snow white solid, melting point 93.5°-96°C. Further elution gave, after a small mixed fraction, pure (+/-)- trans-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane, as a white solid, melting point 93°- 95°C.
A mixture of (+/-)-cis-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane (31.0 g), 4-aminothiophenol (12.6 g) and anhydrous potassium carbonate (23.1 g) in acetone (250 ml) was stirred overnight under reflux under nitrogen. The reaction mixture was then evaporated to dryness, and the resulting residue was extracted with methylene chloride (300 ml) and filtered. The solid filter cake was then washed with methylene chloride (200 ml). The methylene chloride extracts were then combined and concentrated and flash chromatographed on a silica gel eluting with methylene chloride followed by 30% acetone in methylene chloride. The pure product was dissolved in a minimum amount of hot ethyl acetate (125 ml), the solution diluted with an equal volume of hot hexane and seeded to give 30.0 g of (+/-)-cis-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1- yl)methyl-4-(4-amino-phenylthio)methyl-1,3-dioxolane, melting point 121°- 122.5°C.
In practice it is usually used as hydrochloride.
To a solution of 4-(4-chlorophenyl)but-1-ene in dichloromethane was added dropwise with stirring a mixture of 40% peracetic acid and sodium acetate. The resulting mixture was heated under reflux, cooled, and stirred with water. The dichloromethane layer was separated, washed with dilute aqueous potassium carbonate until neutral, water, and dried (MgSO4) and evaporated to give 4-(4-chlorophenyl)-1,2-epoxybutane as a colorless oil.
To a suspension of sodium hydride (50% dispersion in mineral oil) in dry DMF under nitrogen was added imidazole in dry DMF with stirring at such a rate as to keep the temperature below 65°C (ice bath). When the evolution of gas had ceased, 4-(4-chlorophenyl)-1,2-epoxybutane was added dropwise and the mixture stirred overnight at room temperature. The resulting brown solution was added water, extracted with ethyl acetate and the combined extracts were washed with water 3 times, dried (MgSO4) and evaporated to give an oil which crystallized. Washing the solid with ether and filtration give 1-(4-(4- chlorophenyl)-2-hydroxybutyl)imidazole.
A solution of oxalyl chloride (74.8 g) in dry dichloromethane (1350 ml) at below -70°C under a nitrogen atmosphere was treated with dry dimethylsulfoxide (91.5 ml) in methylene chloride (270 ml) dropwise over 15- 20 min while maintaining the temperature of the reaction mixture below - 50°C. After an additional 5 min a solution of (+/)-1-(4-(4-chlorophenyl)-2- hydroxybutyl)imidazole (128.3 g) in a mixture of dimethylsulfoxide (50 ml) and methylene chloride (200 ml) was added over a period of 20 min keeping the reaction mixture at temperatures below -65°C. After a further 15 min dry triethylamine (300 ml) was added rapidly and after 15 min the reaction mixture was allowed to warm to 0°C. Water (20 ml) was then added to the reaction mixture, the methylene chloride then removed by evaporation and the resulting slurry was then treated with water and filtered. The filter cake was washed well with ice water, cold ethyl acetate and then dried in air to yield 118.0 g of 4-(4-chlorophenyl)-1-(imidazol-1-yl)butan-2-one.
A mixture of 4-(4-chlorophenyl)-1-(imidazol-1-yl)butan-2-one (50.0 g), ptoluenesulfonic acid monohydrate (42.07 g) and glycerol (37.0 g) in toluene (200 ml) was heated under reflux, with stirring, through a Dean-Stark trap for 6 h. The two layers were allowed to separate and the hot toluene (upper layer) decanted and discarded. The lower layer was poured into 2 N sodium hydroxide (500 ml), the transfer completed by washing the flask with 1 N sodium hydroxide and methylene chloride, and the product extracted with methylene chloride (4x200 ml). The extracts were dried (MgSO4) evaporated and the residue recrystallized from toluene to give 61.4 g of (cis/trans)-2-(2- (4-chlorophenyl)ethyl)-2-(imidazol-1-yl)methyl-4-hydroxymethyl-1,3- dioxolane, melting point 96°-110°C.
(cis/trans)-2-[2-(4-Chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- hydroxymethyl-1,3-dioxolane (39.7 g) in pyridine (150 ml) at 0°C was treated drop-wise with stirring with methanesulfonyl chloride (10.6 ml) and the mixture stirred overnight. The resulting solid mass was stirred with ether (500 ml) to break up the solid, filtered and washed well with ether to give (cis/trans)-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane hydrochloride, melting point 107°- 110°C (recrystallized from dichloromethane/isopropanol).
The (cis/trans)-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane hydrochloride was basified with aqueous potassium carbonate solution, extracted with ethyl acetate (2x400 ml) and the extracts washed, dried (MgSO4) and evaporated. The resulting semicrystalline mass was chromatographed on silica gel (900.0 g) eluting with dichloromethane, aqueous ethyl acetate (2.2% water) to give 25.4 g of (+/-)- cis-2-(2-(4-chlorophenyl)ethyl)-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane, as a snow white solid, melting point 93.5°-96°C. Further elution gave, after a small mixed fraction, pure (+/-)- trans-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane, as a white solid, melting point 93°- 95°C.
A mixture of (+/-)-cis-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1-yl)methyl-4- (methylsulfonyloxy)methyl-1,3-dioxolane (31.0 g), 4-aminothiophenol (12.6 g) and anhydrous potassium carbonate (23.1 g) in acetone (250 ml) was stirred overnight under reflux under nitrogen. The reaction mixture was then evaporated to dryness, and the resulting residue was extracted with methylene chloride (300 ml) and filtered. The solid filter cake was then washed with methylene chloride (200 ml). The methylene chloride extracts were then combined and concentrated and flash chromatographed on a silica gel eluting with methylene chloride followed by 30% acetone in methylene chloride. The pure product was dissolved in a minimum amount of hot ethyl acetate (125 ml), the solution diluted with an equal volume of hot hexane and seeded to give 30.0 g of (+/-)-cis-2-[2-(4-chlorophenyl)ethyl]-2-(imidazol-1- yl)methyl-4-(4-amino-phenylthio)methyl-1,3-dioxolane, melting point 121°- 122.5°C.
In practice it is usually used as hydrochloride.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine