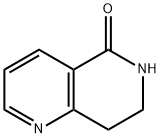
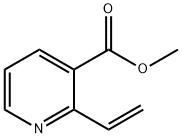
General procedure for the synthesis of 7,8-dihydro-1,6-naphthyridin-5(6H)-one from methyl 2-vinylnicotinate: methyl 2-vinylnicotinate (0.40 g, 2.47 mmol, 1 eq.) was dissolved in acetic acid/water (1:1, 4.6 mL) and ammonium chloride (1.78 g, 33.58 mmol, 14 eq.) was added. The reaction mixture was heated to reflux for 4 hours. Upon completion of the reaction, it was cooled to room temperature, filtered through a diatomaceous earth pad and washed sequentially with EtOAc and EtOAc/MeOH (9:1). The filtrates were combined and concentrated and the residue was purified by column chromatography using EtOAc/MeOH (95:5) as eluent. The target fractions were collected, concentrated and crystallized from EtOAc to afford 7,8-dihydro-1,6-naphthyridin-5(6H)-one (0.10 g, 25% yield) as a white solid. Melting point: 168-171 °C. 1H NMR (300 MHz, CDCl3) δ 8.61 (dd, J = 4.9/1.8 Hz, 1H), 8.29 (dd, J = 7.9/1.8 Hz, 1H), 7.30 (dd, J = 7.6/4.9 Hz, 1H), 7.04 (br s, NH), 3.65 (td, J = 6.7 /2.7 Hz, 2H), 3.18 (t, J = 6.7 Hz, 2H).13C NMR (75 MHz, CDCl3) δ 165.9, 158.8, 152.4, 135.8, 124.8, 122.7, 39.4, 31.0.MS (ESI) m/z 149 (M + H)+.IR (KBr) 2924, 1679, 1590, 1460. 1679, 1590, 1460, 1232, 1068 cm-1. elemental analysis (C8H8N2O) calculated: C, 64.85; H, 5.44; N, 18.91. measured: C, 65.10; H, 5.80; N, 18.72.