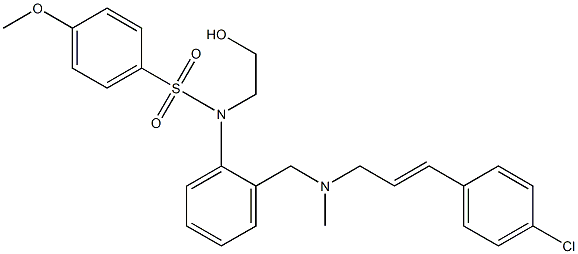
KN-93 is a selective inhibitor of Ca2+/calmodulin-dependent kinase type II (CaMKII), competitively blocking CaM binding to the kinase (Ki = 370 nM). It does not affect the activities of PKA, PKC, MLCK, or Ca2+-phosphodiesterase. It inhibits histamine-induced aminopyrine uptake in parietal cells (IC50 = 300 nM). More recently, KN-93 has been used to implicate roles for CaMKII in Ca2+-induced Ca2+ release in cardiac myocytes, constitutive phosphorylation of 5-lipoxygenase in 3T3 cells, and Ca2+-dependent activation of HIF-1α in colon cancer cells.
white to off-white powder
KN-93 has been used:
- as an inhibitor to block αCaMKII (Ca2+/calmodulin-dependent protein kinase IIα)
- as CaMKII inhibitor to pre-treat hippocampal slices
- to treat the cells, to alter the cytoskeleton (CSK) structure and function during the experiment
A potent and selective inhibitor of Ca2+/Calmoduline-dependent protein kinase II (CaMKII).
ChEBI: A sulfonamide resulting from the formal condensation of p-methoxybenzenesulfonic acid with the anilino nitrogen of 2-(aminomethyl)-N-(2-hydroxyethyl)aniline in which the hydrogens of the primary amino group have been replace
by methyl and p-chlorocinnamyl groups. KN-93 is a selective inhibitor of Ca2+/calmodulin-dependent protein kinase II.
Cell-permeable, reversible, and competitive inhibitor of rat brain Ca+2/calmodulin-dependent protein kinase II (Ki = 370 nM). KN-93 selectively binds to the Calmodulin (CaM) binding site of the enzyme and prevents the association of CaM with CaM Kinase II. Selectively binds to the CaM binding site of the enzyme and prevents the association of CaM with CaMKII. Has no significant effect on protein kinase A activity. Induces G1 cell cycle arrest and apoptosis in NIH 3T3 cells.
Potent inhibitor of CaM kinase II (IC 50 = 0.37 μ M). Also a direct extracellular open channel blocker of voltage-gated potassium channels (IC 50 = 307 nM for Kv1.5); independent of CaM kinase II inhibition.
KN-93 is a selective Ca2+/calmodulin-dependent protein kinase II inhibitor, which has been implicated in the regulation of smooth muscle contractility. CaM kinase II activation was inhibited by KN-93 pretreatment (IC50 ~1 μM). KN-93 inhibited histamine-induced tonic force maintenance, whereas early force development and MLC20 phosphorylation responses during the entire time course were unaffected. Both force development and maintenance in response to KCl were inhibited by KN-93. Rapid increases in KCl-induced MLC20 phosphorylation were also inhibited by KN-93, whereas steady-state MLC20 phosphorylation responses were unaffected. In contrast, phorbol 12,13-dibutyrate (PDBu) did not activate CaM kinase II and PDBu-stimulated force development was unaffected by KN-93. Thus KN-93 appears to target a step(s) essential for force maintenance in response to physiological stimuli, suggesting a role for CaM kinase II in regulating tonic contractile responses in arterial smooth muscle. Pharmacological activation of protein kinase C bypasses the KN-93 sensitive step.