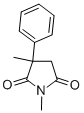
Methsuximide is a succinimide that is converted to N-desmethylmethosuximide, a channel blocker that targets low threshold calcium currents. Methsuximide is a substrate of cytochrome P450 (CYP) isoform 2C19 that, in turn, inhibits CYP2C19-mediated metabolism of biguanides. Methsuximide has been shown to have anticonvulsant properties in clinical trials.
Celontin,Parke Davis,US,1957
A calcium channel succinimide antiepileptic drug. Anticonvulsant.
ChEBI: Methsuximide is an organic molecular entity.
100 g of α-phenyl-α-methylsuccinic acid and 110 g of 40% aqueous methyl amine are heated together at 200 to 250°C until no more distillate is obtained. Upon vacuum distillation of the residue, the N-methyl-α-phenyl-αmethylsuccinimide, of BP 121° to 122°C at 0.1 mm is obtained. After recrystallization from aqueous ethanol, this compound melts at 52° to 53°C.
Although methsuximide is less commonly used, it may be indicated for the control of absence seizures refractory to other drugs.
Although it does not precipitate tonic-clonic convulsions, it often is combined with phenytoin or phenobarbital when absence
seizures coexist with tonic-clonic symptoms. Much of the efficacy of methsuximide is attributed to its desmethyl metabolite. The
half-life of methsuximide is between 2.6 and 4.0 hours, but the half-life for N-desmethylsuximide is 25 hours, causing it to
accumulate substantially. Concentrations of greater than 40 g/mL may be associated with toxicity. Methsuximide is considered
to be more toxic than ethosuximide.
[1] nicholls, p. j., and orton, t.c. the physiological disposition of 14c-methsuximide in the rat. br.j.pharmacol. 45(1), 48-59 (1972).
[2] chen g, weston j k, bratton a c. anticonvulsant activity and toxicity of phensuximide, methsuximide and ethosuximide[j]. epilepsia, 1963, 4(1‐4): 66-76.
[3] sigler m, strassburg h m, boenigk h e. effective and safe but forgotten: methsuximide in intractable epilepsies in childhood[j]. seizure, 2001, 10(2): 120-124.
[4] wright j d, helsby n a, ward s a. the role of s‐mephenytoin hydroxylase (cyp2c19) in the metabolism of the antimalarial biguanides[j]. british journal of clinical pharmacology, 1995, 39(4): 441-444.
[5] guengerich f p. human cytochrome p450 enzymes[m]//cytochrome p450. springer us, 1995: 473-535.