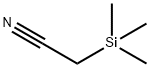
bp 84–85 °C/54 mmHg, 66 °C/35 mmHg,
65–70 °C/20 mmHg;3 d 0.827 g cm?3.
(Trimethylsilyl)acetonitrile has been used in catalytic cyanomethylation of various carbonyl compounds in the presence of Lewis bases.
α-carbanion is a useful alternative to LiCH2CN for nucleophilic
cyanomethylation; (Z)-selective Peterson alkenation). Trimethylsilylacetonitrile participates in the following synthesis reactions: Metalation, Peterson Alkenation (Cyanomethylenation), Michael Addition, Reactions with Other Electrophiles, Cyanomethylation, Metal-Catalyzed Reactions etc.
From chlorotrimethylsilane, zinc, and
XCH2CN, (X = Cl, 61% yield; X = Br, 81% yield).
Check if NMR and IR spectra show impurities; if present dissolve it in *C6H6 (10volumes), wash it with buffer (AcOH/AcONa pH ca 7) several times, dry (CaCl2) it, evaporate and distil it. IR: � (CCl4) 2215 (CN) cm-1, NMR (CCl4): 0.23 (s, 9H, SiMe3), and 1.53 (s, 2H, CH2CN). [Matsuda et al. J Chem Soc, Perkin Trans 1 26 1979, Beilstein 4 IV 3974.]