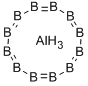3–8μm powder(s); high neutron absorption [ALF93] [HAW93]
Aluminum dodecaboride:
Crystal system, lattice parameters, structure type, Strukturbericht, Pearson, space group, structure type (Z): Tetragonal; a = 1016 pm; c = 1428 pm.
Density (ρ/kg.m–3): 2580; Specific heat capacity (cP/J.kg–1.K–1): 954.48; Vickers or Knoop Hardness (HV or HK/GPa) (/HM): 23.55–
25.50 HK; Other physicochemical: Soluble in hot HNO, insoluble 3
in other acids and alkalis.
Neutron shielding material(uses).
Aluminum dodecaboride is a common form of aluminum boride with higher melting point and hardness, so it is widely studied and applied. Aluminum boride is a significant high-temperature structural material with excellent thermal conductivity and mechanical properties. It finds applications in various sectors such as aerospace, metallurgy, and electronics.
The synthesis method for aluminum dodecaboride involves mixing the appropriate amounts of boron, aluminum, and carbon using equipment such as ball mills. Care should be taken during the mixing process to avoid excessive grinding, as this may negatively impact the subsequent reaction if the raw material particles become too fine. Once mixed, the raw materials need to be dried to remove water and impurities. Subsequently, the dried mixture is placed into a high-temperature furnace for the reaction to occur. The furnace temperature is typically set at around 1600°C, and an inert gas like nitrogen or argon can serve as the reaction atmosphere. During the reaction, a chemical reaction between boron and aluminum takes place, resulting in the production of aluminum dodecaboride. This reaction product gradually forms as the temperature increases.