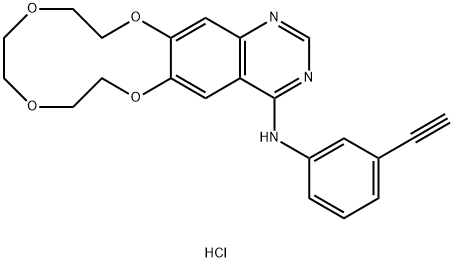
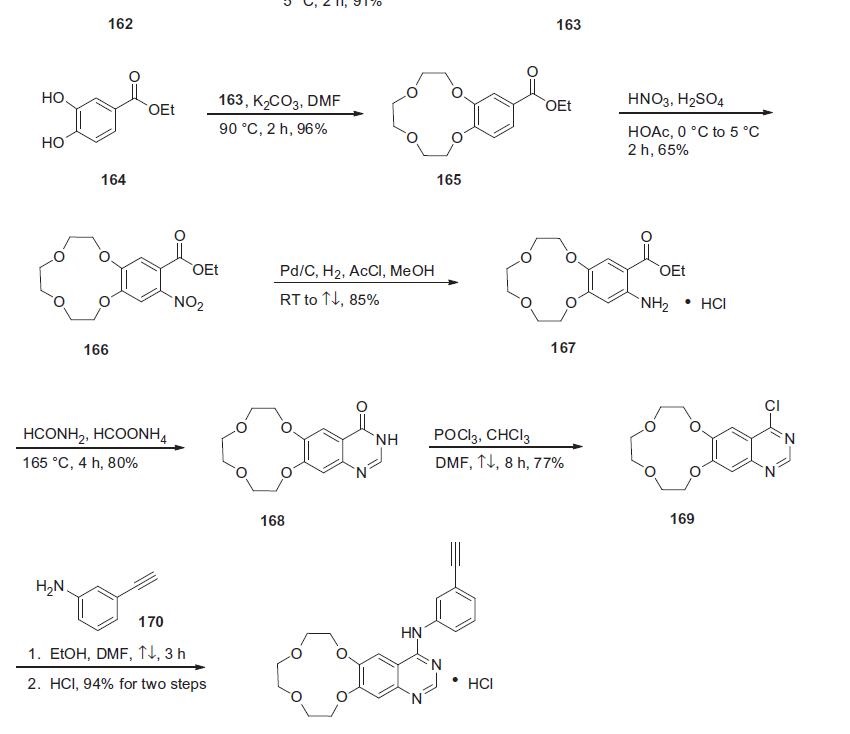
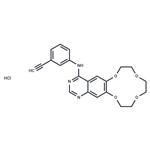
Icotinib hydrochloride, developed by the Chinese pharmaceutical
company Zhejiang Bata Pharma Inc., is a potent small molecule
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor
for the treatment of non-small cell lung cancer (NSCLC). It was first
approved by the SFDA of China, and launched under the brand
name Conmana in the middle of 2011, representing an important
milestone for Chinese pharmaceutical research and development.
As the third EGFR-TKI drug targeting NSCLS therapy, icotinib
hydrochloride possesses a similar structure to gefitinib (Iressa�,
AstraZeneca) and erlotinib (Tarceva, OST & Roche). Interestingly,
a randomized, double-blind phase III clinical study of icotinib
versus gefitinib in 399 patients with advanced NSCLC demonstrated
that icotinib provides similar efficacy to gefitinib, but with
better tolerability in NSCLC patients previously treated with one or
two chemotherapy agents.