A solution of n-butyl lithium in hexane (2.1 M, 9.2 ml, 0.019 mole) is added
dropwise to a solution of diisopropylamine (0.021 mole) in anhydrous
tetrahydrofuran (40 ml) at -40°C (dry ice/isopropanol bath). After standing at
this temperature for 10 minutes, the cold solution is added dropwise to a
stirred, cooled (-60°C) solution of 5-chloro-3-[(1H-imidazol-1-yl)ethyl]-1,2-benzisoxazole (0.019 mole) in anhydrous tetrahydrofuran (40 ml). The
resulting deep red colored reaction mixture is stirred at -60°C for 30 minutes
and then iodomethane (4.1 g, 0.029 mole) is added all at once. The reaction
is stirred at -60°C for 1 hour and then without external cooling for a further 2
hours. Water (100 ml) and ethyl acetate (100 ml) are then added and the
organic solution is separated and extracted with 3 N hydrochloric acid (2x30
ml). The combined acid extracts are subsequently made basic (pH 8) with 2 N
sodium hydroxide and the product is extracted into ethyl acetate (2x50 ml).
The combined extracts are dried over anhydrous sodium sulfate, filtered and
evaporated in vacuum to afford an oil which solidifies on standing. The crude
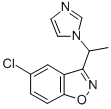
5-chloro-3-[1-(1H-imidazol-1-yl)ethyl]-1,2-benzisoxazole, is recrystallised
from isopropanol, When a warm solution of this material in isopropanol is
treated with a small excess of ethereal hydrogen chloride, the hydrochloride
salt 5-chloro-3-[1-(1H-imidazol-1-yl)ethyl]-1,2-benzisoxazole hydrochloride is
obtained, MP: 168-170°C.