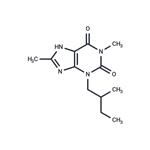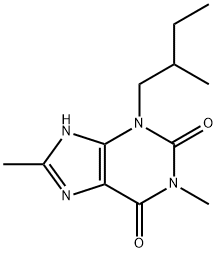
Verofylline
- Product NameVerofylline
- CAS66172-75-6
- MFC12H18N4O2
- MW250.3
- EINECS
- MOL File66172-75-6.mol
Usage And Synthesis
Synthesis of 1,8-dimethyl-3-(2-methyl-1-butyl)xanthine included 7 steps:
1). 1-Methyl-3-(2-methyI-1-butyl)urea:
1.03 kg (11.8 mole) of 2-methyl-1-butylamine was added to 4.5 L of chloroform and the solution cooled to 0-5°C. Then 674.0 g (11.8 moIe) of methyl isocyanate was added slowly while maintaining the temperature at 0.5°C. After the addition was complete the reaction was allowed to reach room temperature. Stirring was continued for 18 hours. The chloroform was removed under vacuum to yield 1.7 kg of 1-methyl-3-(2-methyl-1-butyl)urea as an oil. Yield 100%.
2). 1-Methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea:
To 1.7 kg (11.8 mole) of 1-methyl-3-(2-methyl-1-butyl)urea were added 4.3 L of methyl isocyanate and 1.18 kg (13.9 mole) of cyanoacetic acid. This was heated for 2 hours at 60-70°C. The acetic anhydride was removed under vacuum to yield 2.9 kg of oil. This material is a mixture of cyanoacetic acid and 1-methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea. No attempt was made at purification; the crude oil was used immediately in the next step.
3). 1-Amino-1-methyl-3-(2-methyl-1-butyl)uracil:
10.3 L of 10% NaOH solution was slowly added to 2.9 kg (11.8 mole) of crude 1-methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea with stirring. The oil dissolved and shortly another oil precipitated. The temperature rose to about 60°C and then dropped. After stirring for a while at room temperature the oil crystallized. After cooling the product was filtered. The crude product was slurred in water and dried at 50°C in vacuum to yield 2.1 kg of 4-amino-1- methyl-3-(2-methyl-1-butyl)uracil. MP: 121-124°C. Yield 85%.
4). 4-Amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uracil:
21 kg (9.9 mole) of 4-amino-1-methyl-3-(2-methyl-1-butyl)uracil was suspended in 22.0 L of water. A solution of 745.5 g (10.8 mole) of sodium nitrite in 5.7 L of water was added to the suspension. Then 1.2 L of glacial acetic acid was added dropwise and the suspension was stirred for 18 hours at room temperature. After cooling the precipitate was filtered. The crude product was slurred in water and dried at 80°C in vacuum to yield 1.9 kg of 4-amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uraciI. MP: 202-204°C. Yield 80%.
5). 4,5-Diamino-1-methyl-3-(2-methyl-1-butyl)uracil:
8.65 L of conc. ammonium hydroxide (58%) was added to 1.9 kg (7.9 mole) of 4-amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uracil. An orange salt formed. The suspension was placed in an oil bath at 80-90°C and a solution resulted. 5.6 kg (32.3 mole) of sodium dithionite was added in portions over about 30 min. When the addition was complete stirring was continued for 30 min. The reaction was allowed to cool to room temperature and stirred overnight. After cooling the precipitate was filtered, slurred with water and dried at 80°C in vacuum to yield 1.25 kg of 4,5-diamino-1-methyl-3-(2- methyl-1-butyl)uracil. MP: 161-163°C. Yield 70%.
6). 4-Amino-5-acetylamino-1-methyl-3-(2-methyl-1-butyl)uracil:
1.25 kg (5.5 mole) of 4,5-diamino-1-methyl-3-(2-methyl-1-butyl)uracil was added to 4.5 L of glacial acetic acid and heated to reflux for 2 hours. The acetic acid was evaporated and the residue triturated with ether. The solid was filtered and dried at 60°C in vacuum to yield 1.26 kg of 4-amino-5- acetylamino-1-methyl-3-(2-methyl-1-butyl)uracil. MP: 178-182°C. Yield 85%.
7). 1,8-Dimethyl-3-(2-methyl-1-butyl)xanthine:
1.26 kg (4.7 mole) of 4-amino-5-acetylamino-1-methyl-3-(2-methyl-1- butyl)uracil was added to 3.9 L of 10% sodium hydroxide solution and heated at reflux for 30 min. The solution was filtered and the filtrate cooled to room temperature. The pH of the filtrate was adjusted to 5.0 with glacial acetic acid. After cooling the precipitate was filtered. The crude product was slurred twice with water and dried at 80°C in vacuum to yield about 1.0 kg of 1,8- dimethyl-3-(2-methyl-1-butyl)xanthine. MP: 189-191°C. Yield 85%.
1). 1-Methyl-3-(2-methyI-1-butyl)urea:
1.03 kg (11.8 mole) of 2-methyl-1-butylamine was added to 4.5 L of chloroform and the solution cooled to 0-5°C. Then 674.0 g (11.8 moIe) of methyl isocyanate was added slowly while maintaining the temperature at 0.5°C. After the addition was complete the reaction was allowed to reach room temperature. Stirring was continued for 18 hours. The chloroform was removed under vacuum to yield 1.7 kg of 1-methyl-3-(2-methyl-1-butyl)urea as an oil. Yield 100%.
2). 1-Methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea:
To 1.7 kg (11.8 mole) of 1-methyl-3-(2-methyl-1-butyl)urea were added 4.3 L of methyl isocyanate and 1.18 kg (13.9 mole) of cyanoacetic acid. This was heated for 2 hours at 60-70°C. The acetic anhydride was removed under vacuum to yield 2.9 kg of oil. This material is a mixture of cyanoacetic acid and 1-methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea. No attempt was made at purification; the crude oil was used immediately in the next step.
3). 1-Amino-1-methyl-3-(2-methyl-1-butyl)uracil:
10.3 L of 10% NaOH solution was slowly added to 2.9 kg (11.8 mole) of crude 1-methyl-1-cyanoacetyl-3-(2-methyl-1-butyl)urea with stirring. The oil dissolved and shortly another oil precipitated. The temperature rose to about 60°C and then dropped. After stirring for a while at room temperature the oil crystallized. After cooling the product was filtered. The crude product was slurred in water and dried at 50°C in vacuum to yield 2.1 kg of 4-amino-1- methyl-3-(2-methyl-1-butyl)uracil. MP: 121-124°C. Yield 85%.
4). 4-Amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uracil:
21 kg (9.9 mole) of 4-amino-1-methyl-3-(2-methyl-1-butyl)uracil was suspended in 22.0 L of water. A solution of 745.5 g (10.8 mole) of sodium nitrite in 5.7 L of water was added to the suspension. Then 1.2 L of glacial acetic acid was added dropwise and the suspension was stirred for 18 hours at room temperature. After cooling the precipitate was filtered. The crude product was slurred in water and dried at 80°C in vacuum to yield 1.9 kg of 4-amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uraciI. MP: 202-204°C. Yield 80%.
5). 4,5-Diamino-1-methyl-3-(2-methyl-1-butyl)uracil:
8.65 L of conc. ammonium hydroxide (58%) was added to 1.9 kg (7.9 mole) of 4-amino-5-nitroso-1-methyl-3-(2-methyl-1-butyl)uracil. An orange salt formed. The suspension was placed in an oil bath at 80-90°C and a solution resulted. 5.6 kg (32.3 mole) of sodium dithionite was added in portions over about 30 min. When the addition was complete stirring was continued for 30 min. The reaction was allowed to cool to room temperature and stirred overnight. After cooling the precipitate was filtered, slurred with water and dried at 80°C in vacuum to yield 1.25 kg of 4,5-diamino-1-methyl-3-(2- methyl-1-butyl)uracil. MP: 161-163°C. Yield 70%.
6). 4-Amino-5-acetylamino-1-methyl-3-(2-methyl-1-butyl)uracil:
1.25 kg (5.5 mole) of 4,5-diamino-1-methyl-3-(2-methyl-1-butyl)uracil was added to 4.5 L of glacial acetic acid and heated to reflux for 2 hours. The acetic acid was evaporated and the residue triturated with ether. The solid was filtered and dried at 60°C in vacuum to yield 1.26 kg of 4-amino-5- acetylamino-1-methyl-3-(2-methyl-1-butyl)uracil. MP: 178-182°C. Yield 85%.
7). 1,8-Dimethyl-3-(2-methyl-1-butyl)xanthine:
1.26 kg (4.7 mole) of 4-amino-5-acetylamino-1-methyl-3-(2-methyl-1- butyl)uracil was added to 3.9 L of 10% sodium hydroxide solution and heated at reflux for 30 min. The solution was filtered and the filtrate cooled to room temperature. The pH of the filtrate was adjusted to 5.0 with glacial acetic acid. After cooling the precipitate was filtered. The crude product was slurred twice with water and dried at 80°C in vacuum to yield about 1.0 kg of 1,8- dimethyl-3-(2-methyl-1-butyl)xanthine. MP: 189-191°C. Yield 85%.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine