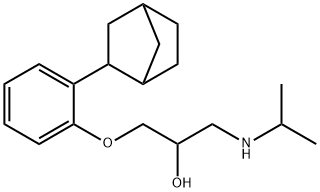
1-Isopropylamino-3-[2-(2-norbornylexo)phenoxy]propan-2-ol:
24.5 g (0.13 moles) 2-(2-norbornylexo)phenol (L. A. KHEIFITS and A. E.
GOL'DOVSKII, Zh. Obshch. Khim., 1963, 33, 2048), 350 ml anhydrous toluene
and 3 g (0.13 mole) metallic sodium are introduced into a three-neck flask
through which a stream of nitrogen flows. The reaction mixture is refluxed
until the liberation of hydrogen ceases, then the solvent is driven off under
reduced pressure and the residue is taken up in 250 ml tetrahydrofuran. 24 g
(0.26 mole) epichlorohydrin are then added and the mixture is heated under
reflux for 6 hours. An extraction with ether is then undertaken, the organic
phase is washed with water, dried and the solvent is evaporated. 25 g 2-(2-
norbornylexo)-1-phenoxy-2,3-epoxypropane are thus obtained in the form of an oil.
15 g (0.06 mole) of the preceding product are dissolved in 50 ml
isopropylamine. After 4 days contact, the excess amine is evaporated under
reduced pressure, then an extraction with ether is carried out. After washing
with water and drying, the ethereal phase is saturated with gaseous
hydrochloric acid. The precipitate formed is washed abundantly with ether
then crystallized from an acetone/ethanol mixture (3/2). 16 g of the desired
product in the form of the hydrochloride are thus obtained, having a melting
point of 189°-191°C.