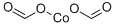cobalt diformate is a red crystalline solid. cobalt diformate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit the spread to the environment. cobalt diformate is used to make catalysts for use in chemical manufacture.
Salts, basic, such as cobalt diformate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
EYES: Causes burns. SKIN: Can cause ulceration.
Behavior in Fire: Decomposes at 175°C.