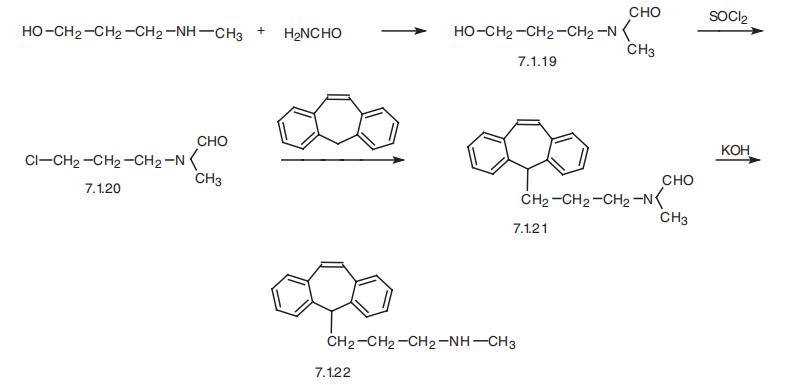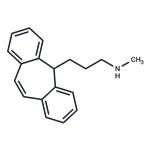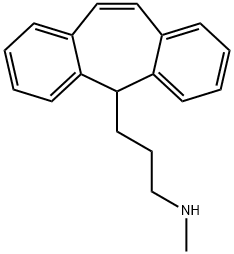
PROTRIPTYLINE
- Product NamePROTRIPTYLINE
- CAS438-60-8
- MFC19H21N
- MW263.38
- EINECS207-119-9
- MOL File438-60-8.mol
Chemical Properties
| Boiling point | 396.62°C (rough estimate) |
| Density | 0.9076 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| pka | 8.2(at 25℃) |
| EPA Substance Registry System | Protriptyline (438-60-8) |
Safety Information
| Hazardous Substances Data | 438-60-8(Hazardous Substances Data) |