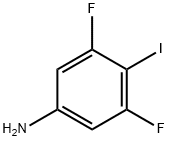
3,5-Difluoro-4-iodoaniline is a halogenated aromatic compound that has a thiol group. It is synthesized by the stepwise alkylation of 3,5-difluoroaniline with ethyl bromoacetate and subsequent deamination with hydrochloric acid. The lithiation process then yields the corresponding lithiated product, which can be iodinated with iodine in acetic acid to give 3,5-difluoro-4-iodoiodobenzenesulfonic acid. The halogenation process involves the treatment of 3,5-difluoro-4-iodoaniline with phosphorus pentachloride to form 3,5-dichloro-4-iodoaniline. Finally, the benzenes are obtained by hydrogenation of the 4th position of this compound.