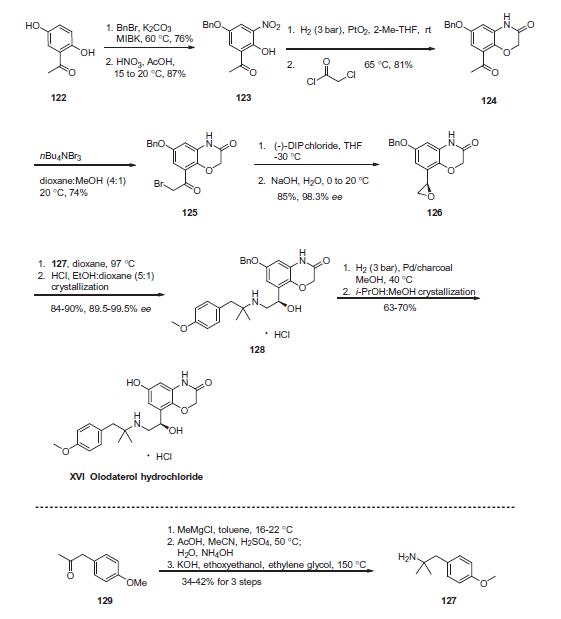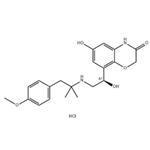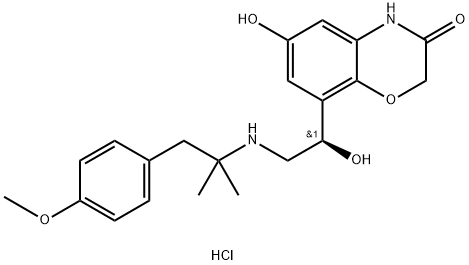
BI 1744 hydrochloride
- Product NameBI 1744 hydrochloride
- CAS869477-96-3
- MFC21H27ClN2O5
- MW422.90248
- EINECS000-000-0
- MOL File869477-96-3.mol
Chemical Properties
| Melting point | 153-155°C |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Off-White to Pale Beige |
| Water Solubility | Water : 250 mg/mL (591.16 mM; Need ultrasonic) |
| Stability | Hygroscopic |
| InChIKey | KCEHVJZZIGJAAW-QAYAHANANA-N |
| SMILES | [C@H](C1C=C(O)C=C2NC(=O)COC=12)(O)CNC(C)(C)CC1C=CC(OC)=CC=1.Cl |&1:0,r| |