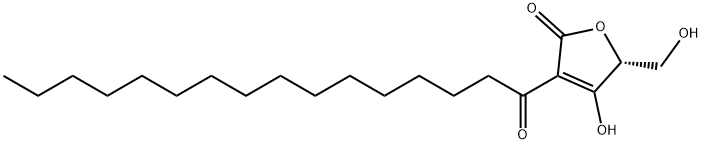
(±)-RK-682 (150627-37-5) is a protein tyrosine phosphatase inhibitor (IC50‘s = 54 7 μM for CD45, 2.0 μM for VHR; did not inhibit cdc25B) originally isolated from the fermentation of Streptomyces sp. 88-682.1?Inhibits cell cycle at G1/S. RK-682 has also been shown to inhibit PLA2 (IC50?= 16 μM)2, HIV-1 protease (IC50?= 84 μlM)3, and heparanase (IC50?= 17 μM)4. Natural RK-682 (R-isomer) and synthetic racemic material have identical phosphatase activity.5?Care should be taken when using RK-682 in the presence of metal salts – because it readily forms metal complexes that affects its phosphatase inhibitory activity.6?RK-682 has been identified as a potential promiscuous inhibitor.7
1) Hamaguchi?et al. (1995),?RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G1?phase; FEBS Lett.,?372?54
2) Shinagawa?et al. (1993),?Tetronic acid derivatives, its manufacturing methods and uses; Jpn.Kokai Tokkyo Koho JP 05-43568,?35?1791
3) Roggo?et al. (1994),?3-Alkanoyl-5-hydroxymethyl tetronic acid homologues and resistomycin; new inhibitors of HIV-1 protease; J. Antibiot (Tokyo),?47?136
4) Ishida?et al. (2004),?Structure-based design of a selective heparanase inhibitor as an antimetastatic agent; Mol. Cancer Ther.,?3?1069
5) Sodeoka?et al1. (1996),?Asymmetric synthesis of RK-682 and its analogs, and evaluation of their protein phosphatase inhibitory activities; Tet. Lett.,?37?8775
6) Sodeoka?et al. (2001),?Asymmetric Synthesis of a 3-Acyltetronic Acid Derivative, RK-682, and Formation of Its Calcium Salt during Silica Gel Chromatography; Chem. Pharm. Bull.,?49?206
7) Carneiro?et al. (2015),?Is RK-682 a promiscuous enzyme inhibitor? Synthesis and in vitro evaluation of protein tyrosine phosphatase inhibition of racemic RK-682 and analogues; Eur. J. Med. Chem.,?97?42