1). 179 mmol of bromine in 20 ml of chloroform were added dropwise in 10
minutes to a solution of 172 mmol of 3-methyl-5-acetylisoxazole, (Gass.
Chim. Ital. 72, 242, 194) containing 4.9 ml of glacial acetic acid while the
reaction mixture was maintained under stirring at 48°-50°C. After 5 minutes
the mixture was poured into 300 g of water and crushed ice. The organic
layer was separated, washed with water, dried and evaporated to residual 3-
methyl-5-bromoacetylisoxazole. Yield 87%, white crystalline compound, MP:
44°-46°C (isopropyl ether).
2). A mixture of 50 mmol of 3-methyl-5-bromoacetylisoxazole and 100 mmol
of thiourea in 164 ml of ethyl alcohol was refluxed for 90 minutes and then
cooled for 1 hour with an ice bath. The precipitate was collected by filtration
and added to a mixture of 25 ml of a 10% aqueous solution of sodium
hydroxide and 100 ml of ethyl acetate under vigorous stirring. The organic
layer was separated, washed, dried and evaporated to dryness after
recrystallization from acetonitrile the 2-amino-4-(3-methyl-5-
isoxazolyl)thiazole melts at 208°-210°C. Yield, 57%.
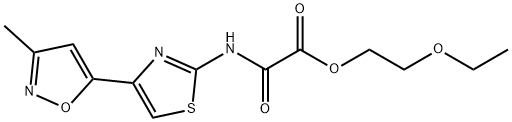
3). To a mixture of 20.5 mmol of 2-amino-4-(3-methyl-5-isoxazolyl)thiazole in
37.4 ml of pyridine maintained under stirring at 5°C were added dropwise
23.6 mmol of 2-ethoxyethyloxalyl chloride. The reaction mixture was
maintained under stirring overnight, then poured into 100 g of crushed ice,
made acid with concentrate hydrochloric acid and extracted with chloroform.
The chloroform extracts were washed with water, dried and evaporated to
dryness. The residual 2-ethoxyethyl-4-(3-methyl-5-isoxazolyl)thiazole-2-
oxamate was re-crystallized from 65 ml of acetonitrile. Yield, 91%, MP: 155°-
156°C.