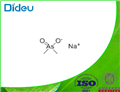
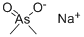Sodium cacodylate is a white crystalline solid which occurs as the trihydrate. It liquefies in the water of hydration @ 60°C and becomes anhydrous @ 120°C.
ChEBI: The organic sodium salt of dimethylarsinate.
A white crystalline or granular solid with a slight odor. Toxic by ingestion, inhalation, and skin absorption. Used as a herbicide.
Sodium cacodylate 97+ gives basic solutions in water. Corrodes common metals, but reaction is not hazardous. [USCG, 1999]. Liquefies in its own water of crystallization when heated to 60°C; becomes anhydrous at 120°C [Merck]. Burns with a bluish flame, emitting a garlic-like odor [Merck].
Toxic by inhalation and ingestion.
Moderately toxic; probable oral lethal dose in humans is 0.5-5 g/kg or between 1 ounce and 1 pint (or 1 lb.) for a 70 kg (150 lb.) person. It may cause disturbances of the blood, kidneys, and nervous system.
Poisoning potential is high when heated to decomposition, or on contact with acids or acid fumes, because Sodium cacodylate 97+ emits highly toxic fumes of arsenic. Avoid water solution in contact with active metals (iron, aluminum, zinc).
Herbicide, Rodenticide, Defoliant, Veterinary medicine: This material has been used as a non-selective herbicide and defoliant for general weed control. Severely restricted for use in EU countries. Not registered for use in the U.S. There are 47 global suppliers
ACME® Sodium cacodylate; ALKARSODYL®; ANSAR 160®; ARSECODILE®; ARSYCODILE®; BOLLS-EYE®; CHEMAID®; DREXEL EZY-PICKIN COTTON DEFOLIANT®; DREXEL KACK HERBICIDE®; DUTCH-TREAT®; HERB-ALL®; PHYTAR 560® (with Cacodylic acid); RAD-E-CATE®; SILVISAR®
This material has been used as a nonselective herbicide and for general weed control.
UN1688 Sodium cacodylate Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3465 Organoarsenic compound, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1Poisonous materials, Technical Name Required
Recrystallise it from aqueous EtOH. [Beilstein 4 H I 612.]
Incompatible with oxidizers, strong bases; acids, active metals (iron, aluminum, zinc). Contact with acids react to form highly toxic dimethylarsine gas. Attacks some metals.
For cacodylic acid, precipitate as calcium arsenate and calcium arsenite by treatment with excess lime water. Recycle if possible. If not, put in secure storage for possible disposal in leach-proof dumps. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.