Alkaloids
Alkaloid is a kind of nitrogen-containing natural compound of physiological function with the majority of them containing complicated heterocyclic structures and a few of them being non-nitrogen heterocyclic organic amine. They generally have similar properties of the alkaline. Some kinds of natural derived vitamins, amino acids and peptides, although also belonging to nitrogen-containing compound, but generally are not classified into the range of alkaloids. In fact the word “alkaloid” still has not yet strict and precise definition to now.
Alkaloid is widely distributed in nature, especially in the plant kingdom. There are also some kinds found in animal kingdom such as the bufotenine found in toad, the hypoxanthine found in the earthworm. According to statistics, it has been found of alkaloids from at least 140 species of plants, especially in poppy, Menispermaceae, Solanaceae, Leguminosae, oleander, Ranunculaceae and barberry Branch. In general, alkaloids are mostly distributed in dicots with less being contained in monocots. There are rarely kinds of alkaloids found in gymnosperms and ferns or even lower plants. Alkaloid-containing plants often contain a variety of alkaloids of similar chemical structure. Plants have close family & genus relationship often contains alkaloids of similar structure while some being the same.
There are several ways of classifications of alkaloids, plant sources, the chemical structure or physiological effect can all be taken as classification references with the later one being more reasonable. Common types include pyrroles, pyridines, quinolines, isoquinolines, indoles, imidazoles, purines, tropane, terpenes, steroids and organic amines. Some people have divided alkaloids into 59 kinds, illustrating the complexity of their structure.
Alkaloids, except for a very few kinds which are liquid at room temperature, mostly appears as crystalline solid. They are generally colorless with bitterness, optically active and mostly left-handed. They exhibit alkalinity with the strength of the alkalinity exhibiting a significant relationship with the molecular structure especially the binding state of the nitrogen atoms. Quaternary ammonium alkaloids exhibit strong alkalinity with tertiary amines and secondary amines alkaloids being moderate or weak alkaline. Some kinds of alkaloids molecules contain phenol groups or carboxyl groups, thus having amphoteric property. The plants alkaloids can mostly bind with organic acid into salt or have condensation reaction with sugar to become glycosides. A few kinds of alkaloids having weak alkalinity exist in their free states. Alkaloid salts, after alkalization or alkaloid glycosides, after hydrolysis can obtain free alkaloid. Most kinds of alkaloids are insoluble or poorly soluble in water, soluble in organic solvents such as benzene, chloroform, ether, acetone, ethanol, and forming salt after being dissolved in dilute acid. Alkaloid salts are more soluble in water and alcohol, insoluble or poorly soluble in organic solvents. Alkaloids can react with some kinds of reagents, such as potassium iodide, bismuth, mercury, potassium iodide, iodine-potassium iodide to form precipitates of different colors. This precipitation reaction is often applied to identify specific alkaloids.
There are many ways for extracting alkaloid. Generally, we can first use water, acid water or alcohol to extract the crushed samples; the extract was concentrated; use organic solvent to extract out the total alkaloids after alkalization. Or first send the crushed sample to alkalization; further apply organic solvent to extract the total alkaloids. The total alkaloids can be further separated through various methods such as chromatography, preparative derivatives. There are few sublimated or volatile alkaloids which can be purified through water steam distillation or sublimation purification.
Though the alkaloid content is very small inside plants, they have intimate relationship with humans. Mankind has long been recognized that some alkaloid-containing plants or crude extracts can be used for treatment of disease or being used as poisoning drugs. Since 1806 when Serturner had isolated morphine from the opium poppy, there have been over 6000 kinds of alkaloids extracted from plants and animals. Among them, nearly 100 kinds have been used or tested in clinical application. Many kinds of alkaloids are effective medicine, such as the berberine contained in Ranunculaceae rhizome has anti-bacterial as well as anti-inflammatory effect; the reserpine in Rauwolfia can lower blood pressure; the galantamine of Lycoris has efficacy in treating post-polio syndrome. The morphine contained in the poppy peel is a kind of famous analgesics; quinine alkaline is a valuable antipyretic drug; Cephalotaxine and vinblastine are effective cancer medicine; colchicine (alkaline) mutagenesis can produce polyploid. Some kinds of alkaloids can be used to make pesticides of agricultural purpose. It has been also isolated in vertebrates and invertebrates of alkaloids, among which, the alkaloids contained in some animals are related to their intake of plant. The alkaloids found in toads, newts and some kinds of fishes are real animal metabolites.
- Structure:
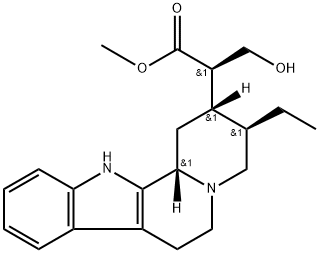
- Chemical Name:(16R)-Dihydrositsirikine
- CAS:6519-26-2
- MF:C21H28N2O3
- Structure:
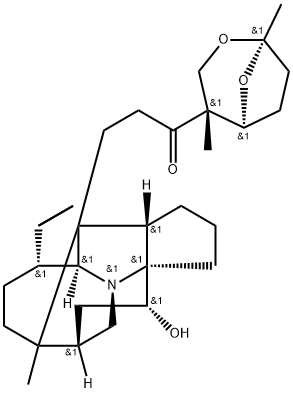
- Chemical Name:11-Hydroxycodaphniphylline
- CAS:1186496-68-3
- MF:C30H47NO4
- Structure:
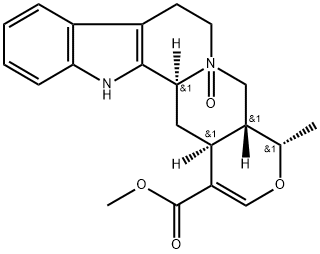
- Chemical Name:4,R-ajmalicine N-oxide
- CAS:41590-29-8
- MF:C21H24N2O4
- Structure:
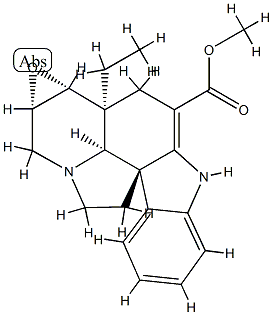
- Chemical Name:(5α,12R,19α)-2,3-Didehydro-6α,7α-epoxyaspidospermidine-3-carboxylic acid methyl ester
- CAS:72058-36-7
- MF:C21H24N2O3
- Structure:
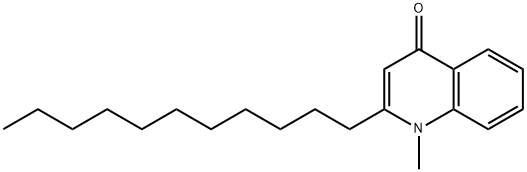
- Chemical Name:1-Methyl-2-undecyl-1,4-dihydroquinoline-4-one
- CAS:59443-02-6
- MF:C21H31NO
- Structure:
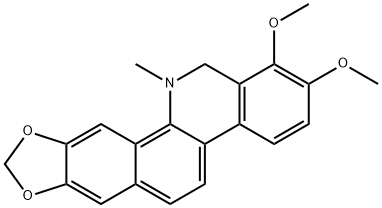
- Chemical Name:Dihydrochelerythrine
- CAS:6880-91-7
- MF:C21H19NO4
- Structure:
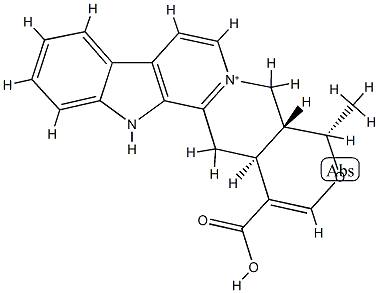
- Chemical Name:Serpentinic acid
- CAS:605-14-1
- MF:C20H19N2O3+
- Structure:
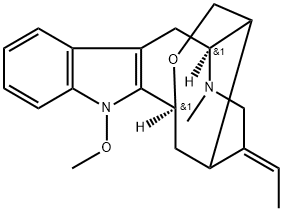
- Chemical Name:N-Methoxyanhydrovobasinediol
- CAS:125180-42-9
- MF:C21H26N2O2
- Structure:
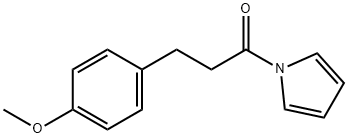
- Chemical Name:3-(4-Methoxyphenyl)-1-(pyrrol-1-yl)propan-1-one
- CAS:448905-82-6
- MF:C14H15NO2
- Structure:
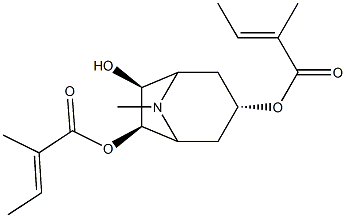
- Chemical Name:3α,6β-Ditigloyloxytropan-7β-ol
- CAS:7159-86-6
- MF:C18H27NO5
- Structure:
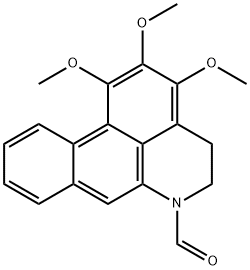
- Chemical Name:DehydroforMouregine
- CAS:107633-69-2
- MF:C20H19NO4
- Structure:
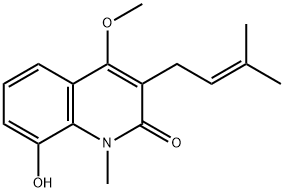
- Chemical Name:Glycosolone
- CAS:67879-81-6
- MF:C16H19NO3
- Structure:
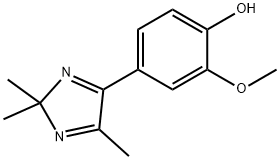
- Chemical Name:Drahebenine
- CAS:1399049-43-4
- MF:C13H16N2O2
- Structure:
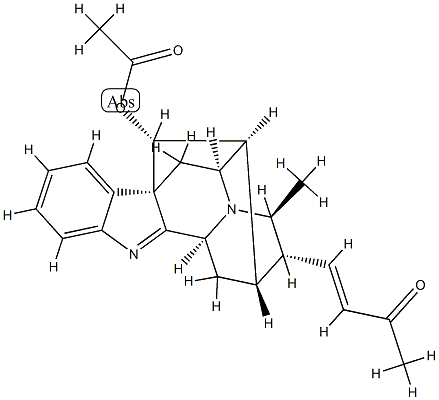
- Chemical Name:Rauvotetraphylline D
- CAS:1422506-52-2
- MF:C24H26N2O3
- Structure:
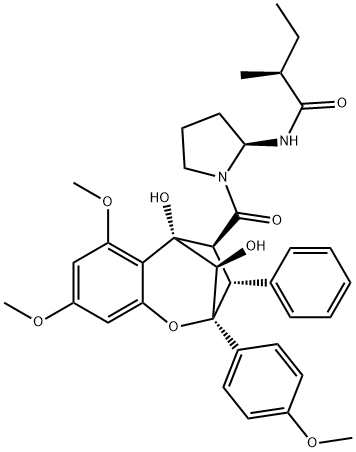
- Chemical Name:Aglain B
- CAS:177262-32-7
- MF:C36H42N2O8
- Structure:
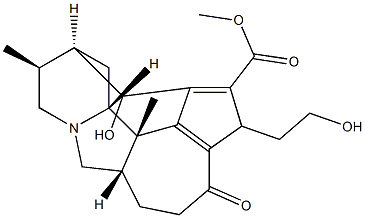
- Chemical Name:Daphnicyclidin H
- CAS:385384-29-2
- MF:C23H29NO5
- Structure:
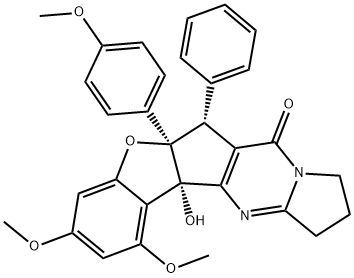
- Chemical Name:Dehydroaglaiastatin
- CAS:155595-93-0
- MF:C31H28N2O6
- Structure:
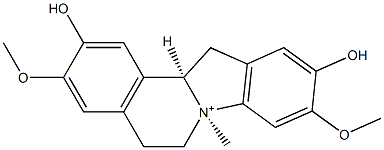
- Chemical Name:Mangochinine
- CAS:209115-67-3
- MF:C19H22NO4+
- Structure:
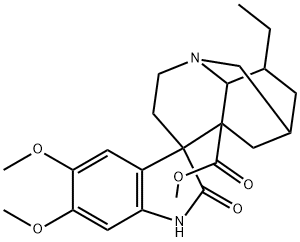
- Chemical Name:(2ξ,4ξ,5ξ,6ξ,18ξ)-Conopharyngine oxindole
- CAS:16790-92-4
- MF:C23H30N2O5
- Structure:
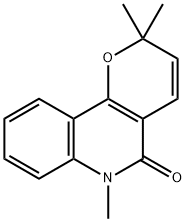
- Chemical Name:2,2,6-Trimethyl-2,6-dihydro-5H-pyrano[3,2-c]quinoline-5-one
- CAS:50333-13-6
- MF:C15H15NO2
- Structure:
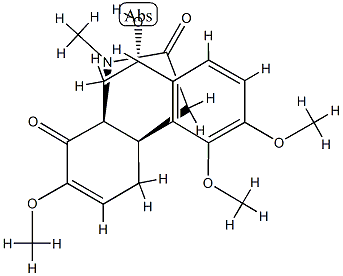
- Chemical Name:16-Oxoprometaphanine
- CAS:58738-31-1
- MF:C20H23NO6
- Structure:
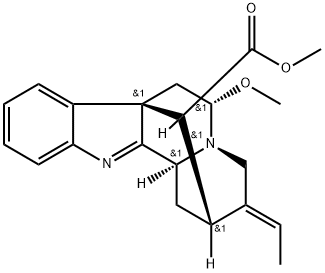
- Chemical Name:5-Methoxystrictamine
- CAS:870995-64-5
- MF:C21H24N2O3
- Structure:
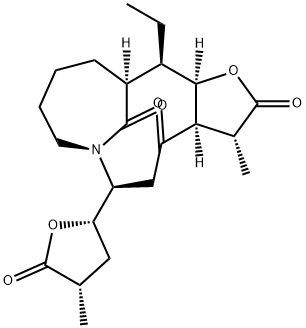
- Chemical Name:Neotuberostemone
- CAS:954379-68-1
- MF:C22H31NO6
- Structure:
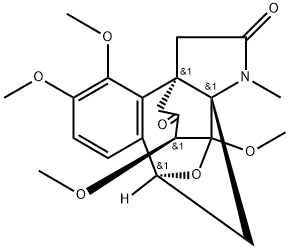
- Chemical Name:Oxoepistephamiersine
- CAS:51804-68-3
- MF:C21H25NO7
- Structure:
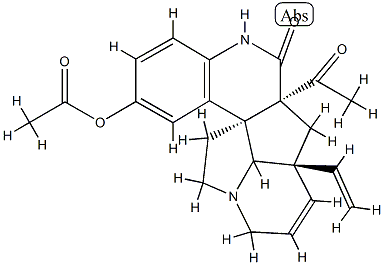
- Chemical Name:10-Acetoxyscandine
- CAS:1432058-90-6
- MF:C23H24N2O5
- Structure:
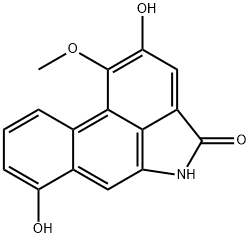
- Chemical Name:AristolactaM AIa
- CAS:97399-90-1
- MF:C16H11NO4
- Structure:
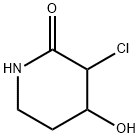
- Chemical Name:3-Chloro-4-hydroxypiperidin-2-one
- CAS:174204-83-2
- MF:C5H8ClNO2
- Structure:
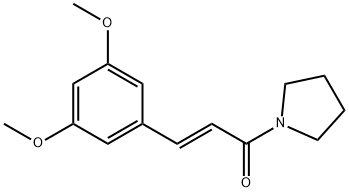
- Chemical Name:4'-DeMethoxypiperlotine C
- CAS:807372-38-9
- MF:C15H19NO3
- Structure:
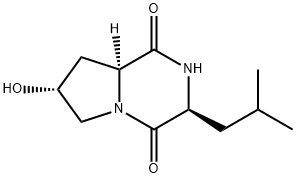
- Chemical Name:Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
- CAS:115006-86-5
- MF:C11H18N2O3
- Structure:
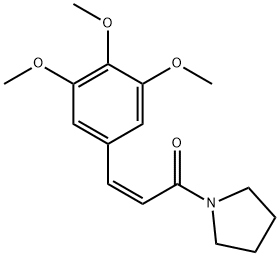
- Chemical Name:Piperlotine D
- CAS:958296-13-4
- MF:C16H21NO4
- Structure:
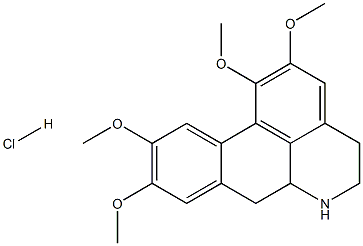
- Chemical Name:Norglaucine hydrochloride
- CAS:39945-41-0
- MF:C20H24ClNO4
- Structure:
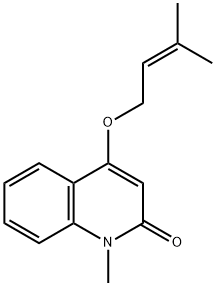
- Chemical Name:Ravenine
- CAS:20105-22-0
- MF:C15H17NO2
- Structure:
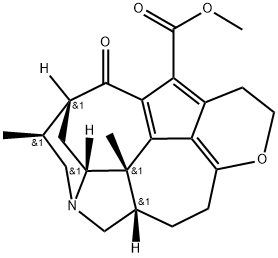
- Chemical Name:Daphnicyclidin D
- CAS:385384-24-7
- MF:C23H27NO4
- Structure:
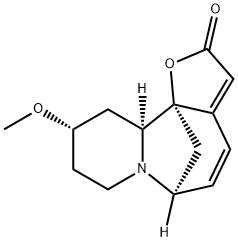
- Chemical Name:Securitinine
- CAS:13861-71-7
- MF:C14H17NO3
- Structure:
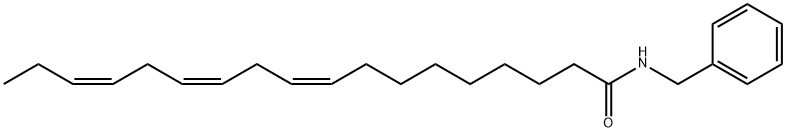
- Chemical Name:N-benzyl-9Z,12Z,15Z-octadecatrienamide
- CAS:883715-18-2
- MF:C25H37NO
- Structure:
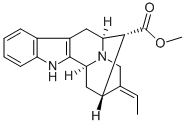
- Chemical Name:pericyclivine
- CAS:975-77-9
- MF:C20H22N2O2
- Structure:
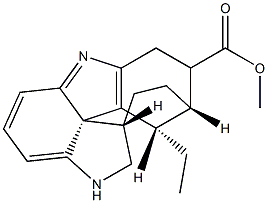
- Chemical Name:tubotaiwine
- CAS:6711-69-9
- MF:C20H24N2O2
- Structure:
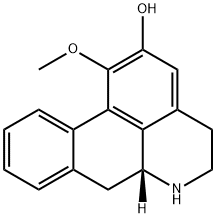
- Chemical Name:asimilobine
- CAS:6871-21-2
- MF:C17H17NO2
- Structure:
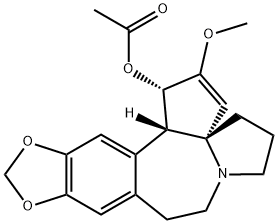
- Chemical Name:Acetylcephalotaxine
- CAS:24274-60-0
- MF:C20H23NO5
- Structure:
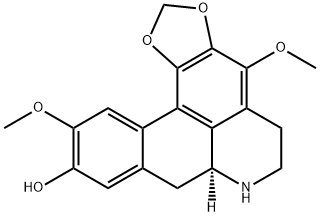
- Chemical Name:Cassyfiline
- CAS:4030-51-7
- MF:C19H19NO5
- Structure:
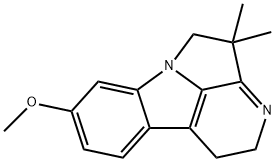
- Chemical Name:Harmalidine
- CAS:109794-97-0
- MF:C16H18N2O
- Structure:
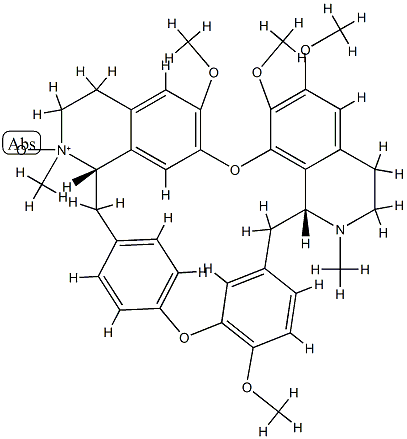
- Chemical Name:Isotetrandrine N-2'-oxide
- CAS:70191-83-2
- MF:C38H42N2O7
- Structure:
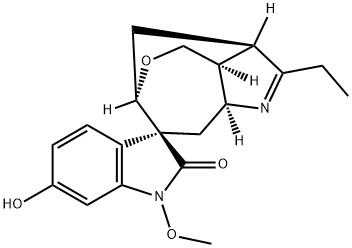
- Chemical Name:11-Hydroxygelsenicine
- CAS:1195760-68-9
- MF:C19H22N2O4
- Structure:
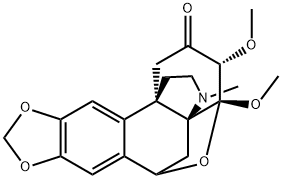
- Chemical Name:Periglaucine B
- CAS:1025023-05-5
- MF:C20H23NO6
- Structure:
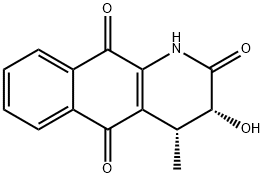
- Chemical Name:Griffithazanone A
- CAS:240122-30-9
- MF:C14H11NO4
- Structure:
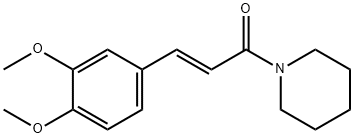
- Chemical Name:1-(3,4-DiMethoxycinnaMoyl)piperidine
- CAS:128261-84-7
- MF:C16H21NO3
- Structure:
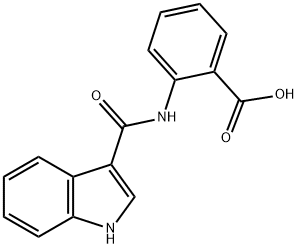
- Chemical Name:2-(1H-Indole-3-carboxaMido)benzoic acid
- CAS:171817-95-1
- MF:C16H12N2O3
- Structure:
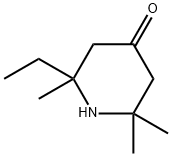
- Chemical Name:2-Ethyl-2,6,6-triMethylpiperidin-4-one
- CAS:133568-79-3
- MF:C10H19NO
- Structure:
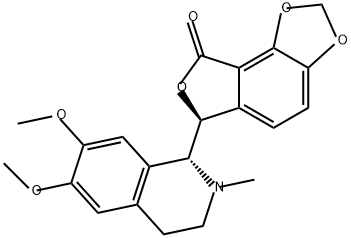
- Chemical Name:(-)-CorluMine
- CAS:79082-64-7
- MF:C21H21NO6
- Structure:
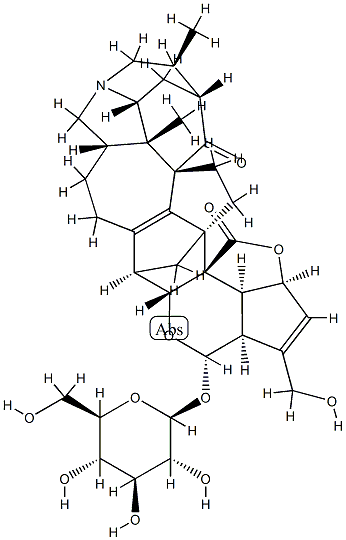
- Chemical Name:Hybridaphniphylline B
- CAS:1467083-09-5
- MF:C37H47NO11
- Structure:
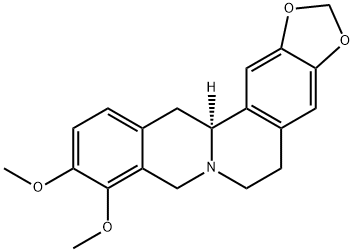
- Chemical Name:canadine
- CAS:5096-57-1
- MF:C20H21NO4
- Structure:
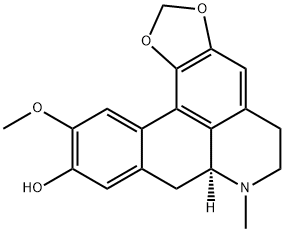
- Chemical Name:(+)-Cassythicine
- CAS:5890-28-8
- MF:C19H19NO4
- Structure:
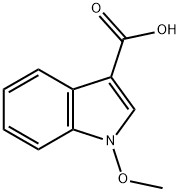
- Chemical Name:1-methoxyindole-3-carboxylic acid
- CAS:91913-76-7
- MF:C10H9NO3
- Structure:
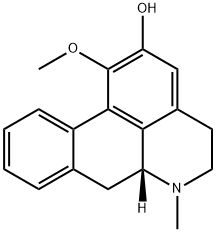
- Chemical Name:ORTHO-NORNUCIFERINE
- CAS:3153-55-7
- MF:C18H19NO2
- Structure:
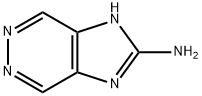
- Chemical Name:zarzissine
- CAS:160568-14-9
- MF:C5H5N5
- Structure:
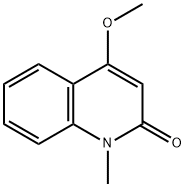
- Chemical Name:4-METHOXY-1-METHYL-2(1H)-QUINOLINONE
- CAS:32262-18-3
- MF:C11H11NO2
- Structure:
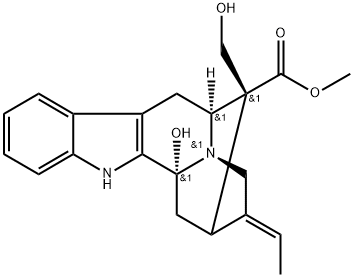
- Chemical Name:16-Epi-voacarpine
- CAS:114027-38-2
- MF:C21H24N2O4
- Structure:
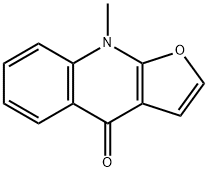
- Chemical Name:9-Methylfuro[2,3-b]quinolin-4(9H)-one
- CAS:484-74-2
- MF:C12H9NO2
- Structure:
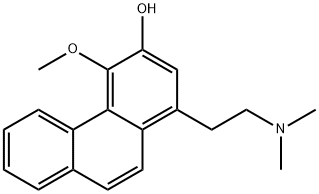
- Chemical Name:1-[2-(Dimethylamino)ethyl]-4-methoxyphenanthren-3-ol
- CAS:16625-57-3
- MF:C19H21NO2
- Structure:
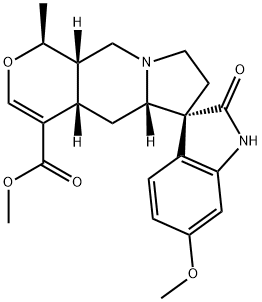
- Chemical Name:11-Methoxyuncarine C
- CAS:61665-08-5
- MF:C22H26N2O5
- Structure:
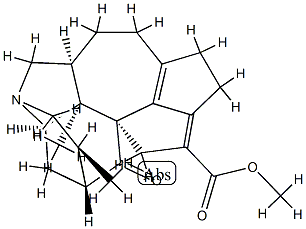
- Chemical Name:Longistylumphylline A
- CAS:857672-34-5
- MF:C23H29NO3
- Structure:
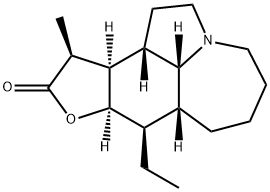
- Chemical Name:Neostenine
- CAS:477953-07-4
- MF:C17H27NO2
- Structure:
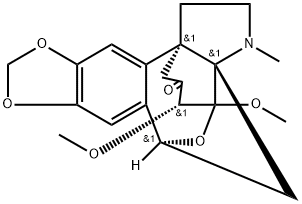
- Chemical Name:Periglaucine A
- CAS:1025023-04-4
- MF:C20H23NO6
- Structure:
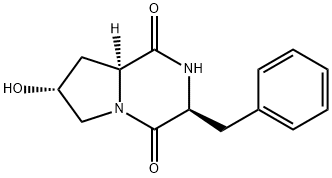
- Chemical Name:Cyclo(L-phenylalanyl-trans-4-hydroxy-L-proline)
- CAS:118477-06-8
- MF:C14H16N2O3
- Structure:
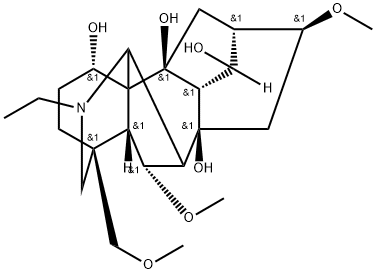
- Chemical Name:10-Hydroxyneoline
- CAS:132362-42-6
- MF:C24H39NO7
- Structure:
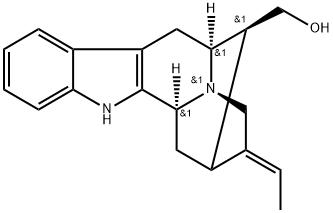
- Chemical Name:16-EpinorMacusine B
- CAS:126640-98-0
- MF:C19H22N2O
- Structure:
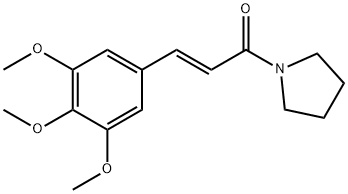
- Chemical Name:Piperlotine C
- CAS:886989-88-4
- MF:C16H21NO4
- Structure:
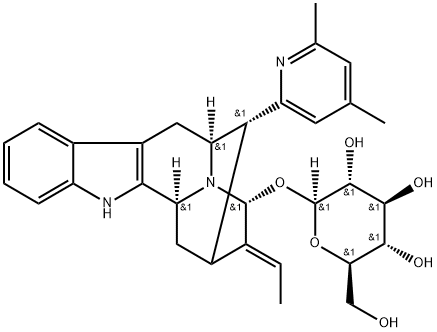
- Chemical Name:Rauvotetraphylline B
- CAS:1422506-50-0
- MF:C31H37N3O6
- Structure:
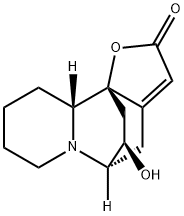
- Chemical Name:Securinol A
- CAS:5008-48-0
- MF:C13H17NO3
- Structure:
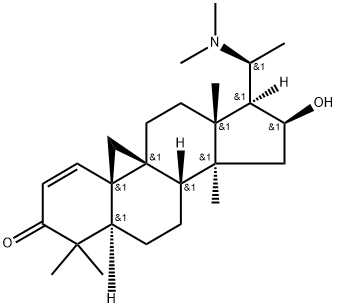
- Chemical Name:Buxbodine B
- CAS:390362-51-3
- MF:C26H41NO2
- Structure:
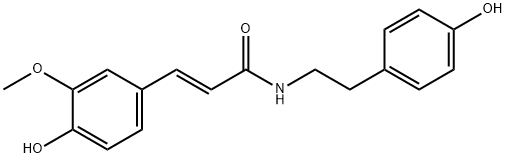
- Chemical Name:N-Trans-Feruloyltyramine
- CAS:66648-43-9
- MF:C18H19NO4
- Structure:
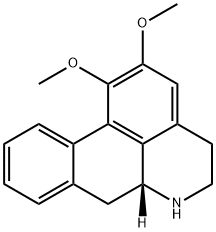
- Chemical Name:(R)-Nornuciferine
- CAS:4846-19-9
- MF:C18H19NO2
- Structure:
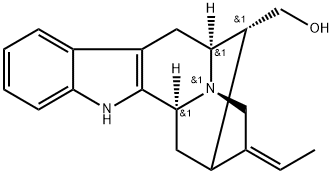
- Chemical Name:(19Z)-Sarpagan-17-ol
- CAS:124096-81-7
- MF:C19H22N2O
- Structure:
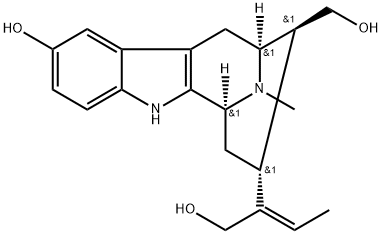
- Chemical Name:Rauvotetraphylline A
- CAS:1422506-49-7
- MF:C20H26N2O3
- Structure:
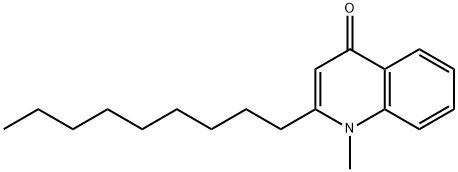
- Chemical Name:1-Methyl-2-nonylquinolin-4(1H)-one
- CAS:68353-24-2
- MF:C19H27NO
- Structure:
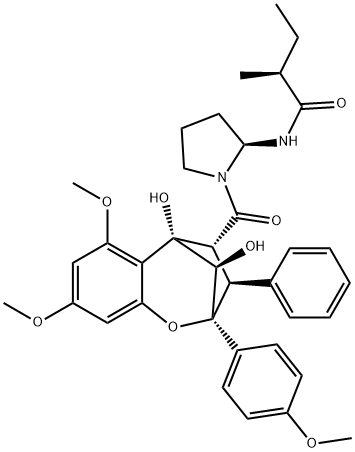
- Chemical Name:Aglain C
- CAS:177468-85-8
- MF:C36H42N2O8
- Structure:
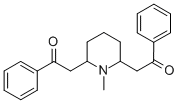
- Chemical Name:2,2'-(1-Methyl-2,6-piperidinediyl)diacetophenon
- CAS:579-21-5
- MF:C22H25NO2
- Structure:
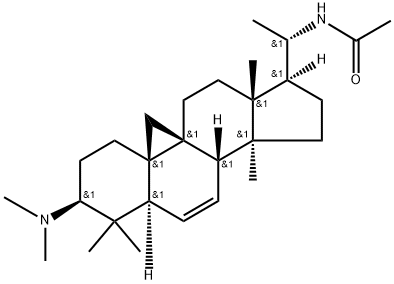
- Chemical Name:Buxbodine D
- CAS:390362-53-5
- MF:C28H46N2O
- Structure:
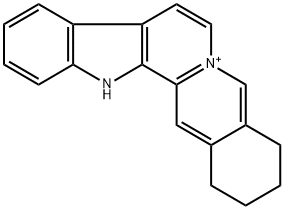
- Chemical Name:sempervirin
- CAS:6882-99-1
- MF:C19H17N2+
- Structure:
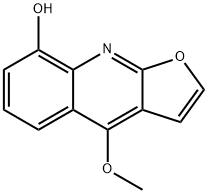
- Chemical Name:4-Methoxyfuro[2,3-b]quinolin-8-ol
- CAS:2255-50-7
- MF:C12H9NO3
- Structure:
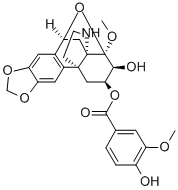
- Chemical Name:8β,10β-Epoxy-8-methoxy-2,3-[methylenebis(oxy)]hasubanan-6β,7β-diol 6-(4-hydroxy-3-methoxybenzoate)
- CAS:33116-33-5
- MF:C26H27NO9
- Structure:
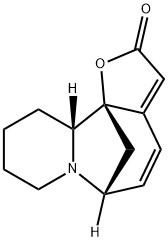
- Chemical Name:(+)-Viroallosecurinine
- CAS:1857-30-3
- MF:C13H15NO2
- Structure:
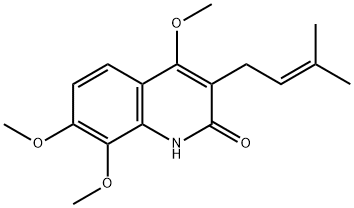
- Chemical Name:4,7,8-Trimethoxy-3-(3-methyl-2-butenyl)quinolin-2(1H)-one
- CAS:38695-41-9
- MF:C17H21NO4
- Structure:
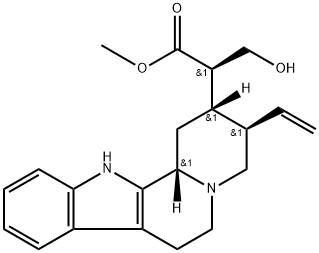
- Chemical Name:(16R)-18,19-Didehydro-17-hydroxycorynan-16-carboxylic acid methyl ester
- CAS:1245-00-7
- MF:C21H26N2O3
- Structure:
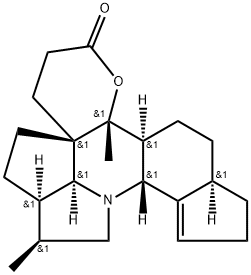
- Chemical Name:Deoxycalyciphylline B
- CAS:619326-74-8
- MF:C22H31NO2
- Structure:
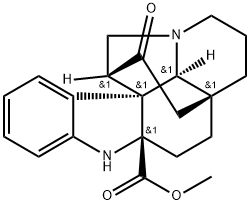
- Chemical Name:Methyl demethoxycarbonylchafruticosinate
- CAS:80151-89-9
- MF:C21H24N2O3
- Structure:
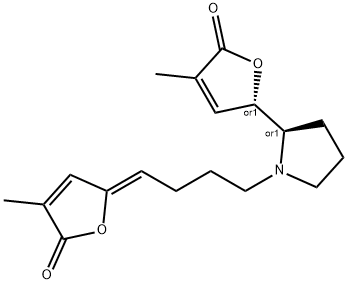
- Chemical Name:Pandamarilactonine B
- CAS:303008-81-3
- MF:C18H23NO4
- Structure:
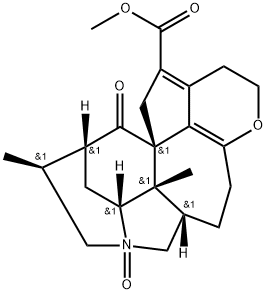
- Chemical Name:Paxiphylline E
- CAS:1092555-03-7
- MF:C23H29NO5
- Structure:
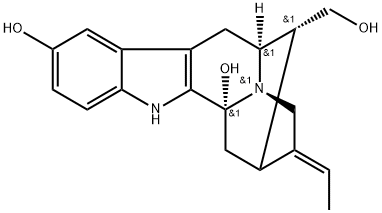
- Chemical Name:3-Hydroxysarpagine
- CAS:857297-90-6
- MF:C19H22N2O3
- Structure:
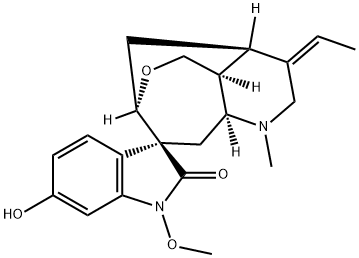
- Chemical Name:11-HydroxyhuMantenine
- CAS:122590-04-9
- MF:C21H26N2O4
- Structure:
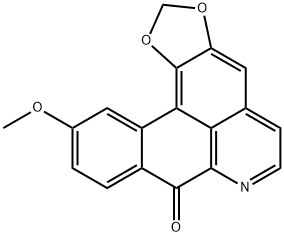
- Chemical Name:Lauterine
- CAS:28200-65-9
- MF:C18H11NO4
- Structure:
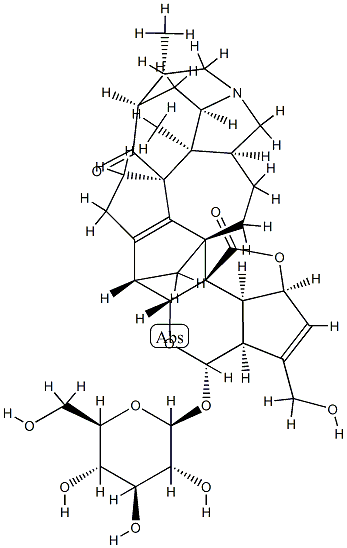
- Chemical Name:Hybridaphniphylline A
- CAS:1467083-07-3
- MF:C37H47NO11
- Structure:
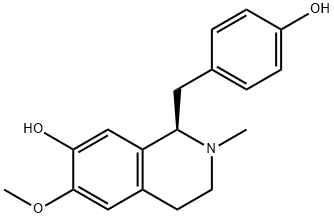
- Chemical Name:N-Methylcoclaurine
- CAS:5096-70-8
- MF:C18H21NO3
- Structure:
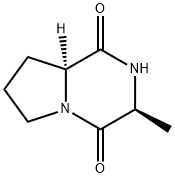
- Chemical Name:Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-methyl-, (3S,8aS)- (9CI)
- CAS:36357-32-1
- MF:C8H12N2O2
- Structure:
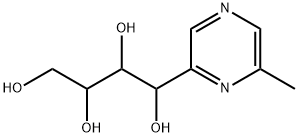
- Chemical Name:pedatisectine F
- CAS:206757-32-6
- MF:C9H14N2O4
- Structure:
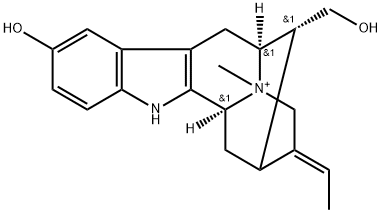
- Chemical Name:spegatrine
- CAS:47326-53-4
- MF:C20H25N2O2+
- Structure:
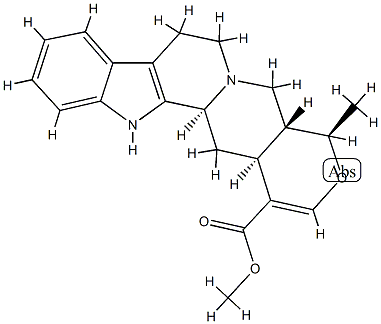
- Chemical Name:16,17-Didehydro-19β-methyl-18-oxayohimban-16-carboxylic acid methyl ester
- CAS:25532-45-0
- MF:C21H24N2O3
- Structure:
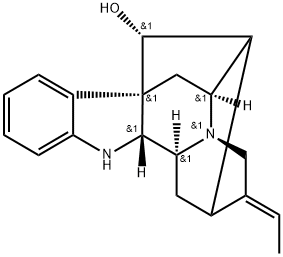
- Chemical Name:(17R,19E)-19,20-Didehydro-1-demethylajmalan-17-ol
- CAS:68160-76-9
- MF:C19H22N2O
- Structure:
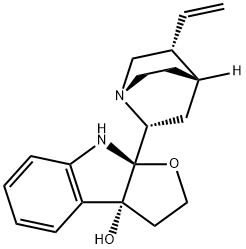
- Chemical Name:conquinamine
- CAS:464-86-8
- MF:C19H24N2O2
- Structure:
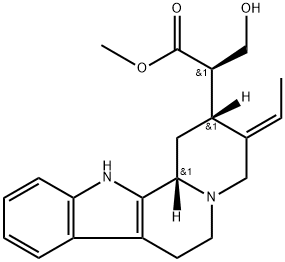
- Chemical Name:16-epi-isositsirikine
- CAS:6519-27-3
- MF:C21H26N2O3