Germicide
Germicide refers to chemicals that can kill or inhibit the bacteria or fungi. Germicide can kill or inhibit the growth and proliferation of pathogens at both outside the plant or inside the plants via the toxicity of the agents. Some kinds of germicides are non-toxic to the fungi, but can interfere with the process of pathogenic process of fungus or affect the interaction of pathogen – host, improving the plant defenses.
In the end of 18th century to the 1850s, people strengthened the research on the organic germicide in order to seek the substitutes of copper and mercury preparations. The event of the greatest impact should be in 1934 when W. H. Tisdale reported the bactericidal effect of dithiocarbamic acid derivatives. This discovery opened up a new era of organic compounds as germicide. Following the discovery of bactericidal activity of ziram, ferbam, and thiram, in 1935, DuPont had further found the germicidal activity of sodium dithane in the dithane class. In 1943, people had put it into production. After 1960s, dithiocarbamate salt germicide had gradually developed into a class of germicide of the world's largest production.
To date, there are nearly 300 kinds of organic germicides that have been commercialized. Substituted benzene type contains PCNB, hexachlorobenzene, chlorothalonil and dozens of other varieties. Trichloromethylthio type germicide mainly contains folpet and captan. After 1950s, there are a lot of varieties having achieved practical application, including organic mercury and quinones, organic tin, organic phosphorus and agricultural antibiotics. In 1969 and 1970, the ethyl thiophanate and methyl thiophanate developed by Nippon Soda Company (Japan) are two best varieties with the latter one especially obtaining wide applications on fruit trees and vegetables. In the 1960s, Japan, during the development of germicide against rice sheath blight, had successfully launched organic arsenic germicides such as asomate and neoasozin. It is particularly worth mentioning that there have been a number of excellent germicides in the heterocyclic fungicides.
The breakthrough of systemic germicide was actually started from the discovery of the systemic germicidal effect of carboxin made by the Uniroyal Company in 1960s. In 1966, carboxin and oxycarboxin had been simultaneously subject to commercialization. Later, it had successively appeared of benomyl, dodecyl morpholine, thiophanate-methyl, and triforine, etc. In 1970s, triazole-class systemic germicide with triadimefon as the representative had attracted broad attention.
However, the above germicides have very poor efficacy in treating many kinds of important diseases caused by oomycete. In 1977, Ciba-Geigy Company (Switzerland) had successfully developed systemic germicide, metalaxyl with excellent efficacy in prevention of disease caused by oomycete. Metalaxyl is characterized by high efficacy, small usage amount and having bidirectional conduction properties, making the systemic germicide enter into a new stage of development. China is one of the earliest countries that had applied the elements and inorganic agents for control of plant diseases. This had been documented (see the history of pesticide development) in a variety of well-known ancient writings. In 1950s, the most widely used germicides are still inorganic copper and mercury preparations.
Substituted benzene such as PCNB has also been applied. In 1960s dithiocarbamate salts and organic arsenic preparations had been widely used. In 1970s, it had been developed of carbendazim, and had developed into one of the germicide varieties with the largest production amount in China. Meanwhile, China's agricultural antibiotic, Jinggangmycin had also obtained widespread application in controlling the Rice Sheath Blight. Since the 1980s, many excellent germicides including organicphosphorus kitazine, fosetyl; heterocyclic germicides such as triadimefon, tricyclazole and isoprothiolane; substituted benzenes germicides such as methyl thiophanate, chlorothalonil and metalaxyl have been popularized.
- Structure:
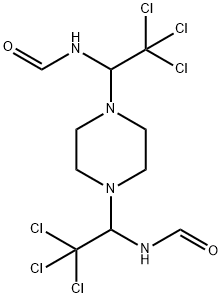
- Chemical Name:TRIFORINE
- CAS:26644-46-2
- MF:C10H14Cl6N4O2
- Structure:
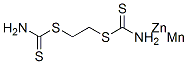
- Chemical Name:Mancozeb
- CAS:8018-01-7
- MF:C4H8MnN2S4Zn
- Structure:
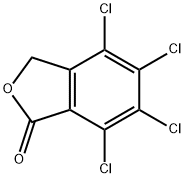
- Chemical Name:FTHALIDE
- CAS:27355-22-2
- MF:C8H2Cl4O2
- Structure:
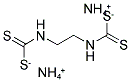
- Chemical Name:AMOBAM
- CAS:3566-10-7
- MF:C4H14N4S4
- Structure:
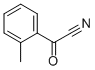
- Chemical Name:2-METHYLBENZOYL CYANIDE
- CAS:5955-73-7
- MF:C9H7NO
- Chemical Name:Carbendazim+Thiram+Sulfur,W.P.
- CAS:
- MF:
- Chemical Name:Carbendazim+Diniconazole,W.P.
- CAS:
- MF:
- Structure:
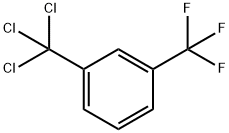
- Chemical Name:3-TRIFLUOROMETHYL BENZOTRICHLORIDE
- CAS:16766-90-8
- MF:C8H4Cl3F3
- Structure:
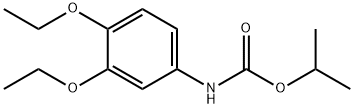
- Chemical Name:Diethofencarb
- CAS:87130-20-9
- MF:C14H21NO4
- Structure:
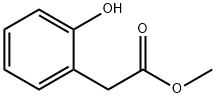
- Chemical Name:(2-HYDROXY-PHENYL)-ACETIC ACID METHYL ESTER
- CAS:22446-37-3
- MF:C9H10O3
- Structure:
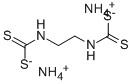
- Chemical Name:AMOBAM
- CAS:3366-10-7
- MF:C4H14N4S4
- Structure:
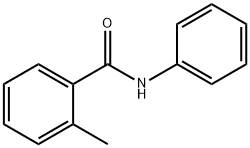
- Chemical Name:2-METHYLBENZANILIDE
- CAS:7055-03-0
- MF:C14H13NO
- Chemical Name:YEKUZUO
- CAS:
- MF:
- Structure:
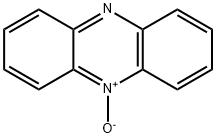
- Chemical Name:PHENAZINE-N-OXIDE
- CAS:304-81-4
- MF:C12H8N2O
- Chemical Name:copper sulfate-ammonia complex
- CAS:
- MF:
- Chemical Name:bouillie bordelaise
- CAS:
- MF:
- Structure:
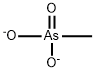
- Chemical Name:Zinc methanearsonate
- CAS:51952-65-9
- MF:CH3AsO3(-2)
- Chemical Name:TTCA wettable powder
- CAS:
- MF: