Triazine herbicides
Triazine herbicide is a kind of broad-spectrum herbicide with the basic chemical structure of triazine. Based on whether the N atom is evenly distributed in phenyl ring or not, it can be divided into even triazine and non-even triazine, two categories. Now it is mainly triazine with its general formula of chemical structure being as follows:

Figure 1 is the general formula of the chemical structure of triazine; X = Cl, SCH3, OCH3, etc; R1, R2, R3 = H, lower alkyl or alkenyl group; R4 = low alkyl or alkenyl group.
A Brief History
In 1952, A. Gast et al had first discovered the herbicidal activity of chlorazine. Later in 1956, people had found that high herbicidal activity of simazine, which was further developed and manufactured by Seigy Company (Switzerland). Since then, triazine herbicide had achieved rapid development among which atrazine has the greatest yield, still remaining as one of the important species in the worldwide herbicide products. In recent years, the development trend of such compounds are: improved processing formulations, improved application techniques, further improving the efficacy and dose savings; and used in combination with alachlor, acetochlor, butachlor, prometryne and cyanazine, etc, improving the weed control efficacy in cornfield and reducing the pollution of groundwater sources. In 1971, it has found of non-even triazine herbicide, metribuzin. The three-nitrogen benzene ring and connect to the heterocyclic sulfonylurea bridge and ortho-substituted phenyl ring to form a new type of high-efficient herbicides such as chlorsulfuron. The prominent advantage is the broad spectrum herbicide and small dosage.
Basic characteristics
The water solubility of such agents has "Tone" type be of the largest, followed by "Zine" category and "Tryne" class is of the lowest. Most triazine type herbicides have relatively stable property, thus having long-term persistence, low toxicity to humans and animals and low toxicity to fishes as well. All of them has systemicity and can be quickly absorbed by the roots after the soil treatment, and transported upward to the blade starting from xylem through the transpiration flow while the regents which can be absorbed by the blade have almost no conducting property, among which simazine and other "Tryne" category herbicidal agents are absorbed by the roots while only atrazine has strong capability of only being subject to foliar uptake. The “Tryne” class herbicides such as prometryn are instead easier to be absorbed from the root, stem and leaf with fast-acting property and strong herbicidal activity on weeds just unearthed. They are easily to be decomposed in the soil, so the residue duration is short with generally lasting 1 to 2 months. "Tone" category has the largest water solubility as well as the strongest herbicidal activity but a poor selectivity.
Mechanism of action
The major mechanism is to inhibit the photosynthesis, affecting the synthesis of the assimilation product. For example, the order of the several inhibitory effects of propazine, prometon, and prometryn on the metabolic processes of bean is photosynthesis, lipid synthesis, RNA synthesis and protein synthesis. Chlorophyll may be the major pigment on which triazine herbicide exerting its toxic effect. The degree of toxicity is directly proportional to the light intensity and the herbicide doesn’t work in the dark. The toxicity is the strongest upon a wavelength of 428 and 658 nanometers. Even triazine herbicide blocks the stream of electrons from water from to the chlorophyll, causing oxidation of chlorophyll, leading to the gradually destruction of the layered structure of chloroplasts. In addition, the herbicide can inhibit the electron transport system during photosynthesis, causing the accumulation of nitrate inside the blade, strengthening the damage to the sensitive plant. Chlorosis is the main symptom of this class of herbicides, first the blade tip will get chlorosis and withered, the leaf edge will then turn yellow, and finally the whole plant dead.
The selectivity of triazine herbicide comes from three cases.
1. the differences in the plant genetics; the order of the drug resistance of cultivated crops to atrazine and simazine is: corn, millet, potato, sorghum, soybeans, millet and sugar beet;
2. selection on potential difference;
3. biochemical detoxification: resistant crops are capable of degrading the triazine herbicides in vivo, so being relatively safe. The most prominent example is the benzoxazinone existing inside the corn body is able to cause the hydroxylation and inactivation of atrazine; triazine herbicide can also from conjugate with the glutathione inside the resistant crops so that the crops get detoxification. Triazine herbicide can also undergo photolysis. In the soil, it can also get dehalogenation, N- dealkylation, deamination and ester hydrolysis, pyrolysis and other reactions to be degraded.
Classification
Based on the difference of the substitution group X in the basic ring and the features of the English name suffix, they are divided into: -Cl substitution group; English nouns suffix is -zine with the Chinese general term being "津"; -SCH3 substitution group: “-tryne” with the Chinese general term being “净”; -OCH3 substitution group; is “tone” type known as" 通 " in Chinese. The currently developed triazine herbicide are mostly even triazine (N atoms are uniformly distributed on the phenyl ring); important non-triazine includes metribuzin.
Application
Triazine herbicides can be applied to various kinds of upland crops with some varieties in the "tryne" category being also be used in rice fields.

Figure 2 is a triazine herbicide and apply crop.
Triazine herbicide mainly targets on the week and bud with no lethal effect on seed as well as no effect on its germination. Atrazine and cyanazine can also be used for stem, leafs and soil treatment in the weed trefoil stage of postemergence, giving a better efficacy. The strongest drug resistance to the triazine herbicide has been observed in corn, millet, sugar cane and apples, grapes. Some varieties of sorghum, carrots, celery, potatoes, cotton, sunflower, peas and soybeans also have strong resistance to them. We can also take advantage of the potential difference or use protective cover in the sprayer to enhance the security of some sensitive plants. "Tryne" class has a general long persistence. After the arrangement of crop of second stubble, we should pay attention to, or reduce the amount of, or be mixed with other agents. The efficacy of using simazine for treatment of weeds belonging to Digitaria and Panicum is better than atrazine. It is relatively safe to apply atrazine for weed control in sorghum field. At the same time, we can apply soil mixture before sowing and apply pre- or post-emergence treatment after sowing. "Tryne" category herbicides have a strong activity on the treatment of the stems and leaves; therefore it can be applied to stem-leaf and soil treatment during the early post-emergence. Sugarcane has a strong resistance without strict requirement on the varieties. Orchard administration of atrazine and prometryne etc. should be performed before the rainy season with high efficacy in the rainy season.
Because atrazine has a relative large water solubility and large mobility in soil as well, long-term high-dose administration can be susceptible to be leached into deep layer of the soil by the layer, affecting the quality of the underground water. Moreover, atrazine has negative effect on the stomach, kidney and liver as well as the genetic material DNA of mammalian. Some countries such as Germany have been discontinued or controlled its administration. China has begun to take measures of mixing it together with alachlor and butachlor to slow the progression of their shortcomings.
- Structure:
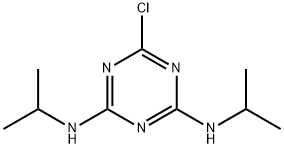
- Chemical Name:2,4-Bis(isopropylamino)-6-chloro-1,3,5-triazine
- CAS:139-40-2
- MF:C9H16ClN5
- Structure:
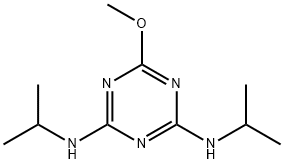
- Chemical Name:PROMETON
- CAS:1610-18-0
- MF:C10H19N5O
- Structure:
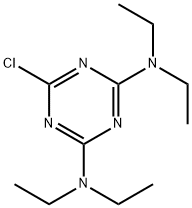
- Chemical Name:chlorazine
- CAS:580-48-3
- MF:C11H20ClN5
- Structure:
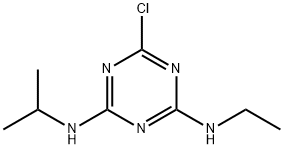
- Chemical Name:Atrazine
- CAS:1912-24-9
- MF:C8H14ClN5
- Structure:
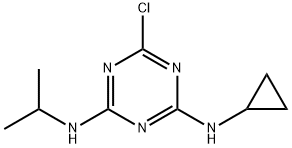
- Chemical Name:CYPRAZINE
- CAS:22936-86-3
- MF:C9H14ClN5
- Structure:
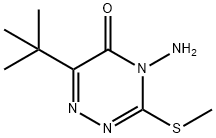
- Chemical Name:Metribuzin
- CAS:21087-64-9
- MF:C8H14N4OS
- Structure:
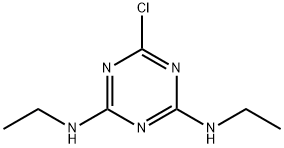
- Chemical Name:Simazine
- CAS:122-34-9
- MF:C7H12ClN5
- Structure:
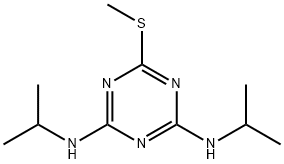
- Chemical Name:Prometryn
- CAS:7287-19-6
- MF:C10H19N5S
- Structure:
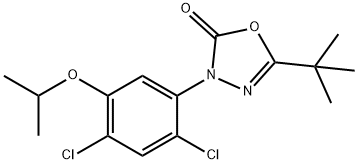
- Chemical Name:Oxadiazon
- CAS:19666-30-9
- MF:C15H18Cl2N2O3
- Structure:
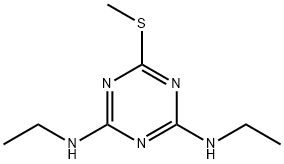
- Chemical Name:Simetryne
- CAS:1014-70-6
- MF:C8H15N5S
- Structure:
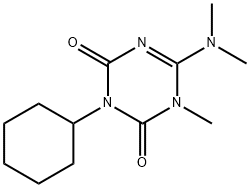
- Chemical Name:Hexazinone
- CAS:51235-04-2
- MF:C12H20N4O2
- Structure:
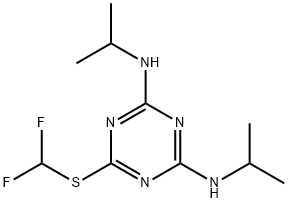
- Chemical Name:SSH-108
- CAS:103427-73-2
- MF:C10H17F2N5S