Mitogen-activated protein kinase (MAPK)
Mitogen-activated protein kinase (MAPK) refers to a class of conserved serine/threonine kinase that being ubiquitously presented in eukaryotes. MAPK cascade pathway (MAPKKK-MAPKK-MAPK) is capable of transducing environmental signals through sequential phosphorylation, participating in regulating plant growth and development as well as a variety of biotic and abiotic stress signal transduction. MAPKs activation pathway plays a crucial role in the signal transduction process in eukaryotic cells. In 1982, during the study of the platelet-derived growth factor and epidermal growth factor, cooper found that a kind of intracellular protein (with relative molecular mass of 42 × 103) was subject to phosphorylation in its tyrosine residues. He then (in 1988) also found this protein in PMA-stimulated cells through two-dimensional electrophoresis.
At the same time, Ray had also successfully isolated a protein kinase of a molecular weight of 42X103 with its threonine and tyrosine residues being phosphorylated from the 3T3-L1 cells and named it MAPKs. Rossomando (1989) have clarified that this kind of protein that subjects to insulin-stimulated tyrosine / threonine residue phosphorylation is the same protein as the protein that subjects to tyrosine residue phosphorylation due to the stimulation of other growth factors and PMA; threonine / tyrosine residues double phosphorylation is a necessary condition for activation of this protein. Boulton (in 1990) had first cloned the cDNA encoding MAPKs; Crews (In 1993) had successfully identified the upstream kinase (MKKKs and MKKs) of MAPKs in mammalian cells by biochemical and molecular cloning techniques and defined a conservative three-kinase activation mode.
The composition of the MAPKs activation pathway
MAPKs activation pathway can be activated by a variety of factors including growth factors, cytokines, radiation, neurotransmitters, hormones and cell stress, etc. MAPKs activation pathway includes three sequentially activated protein kinase: MKKKs → MKKs → MAPKs. It has been found from mammalian cells of at least 14 kinds of MKKKs, 7 kinds of MKKs and 12 kinds of MAPKs.
(1) MKKKs: MKKKs is a kind of serine / threonine protein kinase. Specific MKKKs can be either activated through phosphorylation mediated by its MKKKs kinase (MKKKKs) or activated through the interaction with Ras or the small G protein in Rho family.
(2) MKKs: Gartner et al have confirmed, MKKs can recognize MAPKs to activate the threonine X tyrosine domain inside the ring, causing phosphorylation of the threonine and tyrosine residues and further being activated. Therefore, MKKs is a kind of protein kinase of dual specificity.
(3) MAPKs: MAPKs are a class of protein kinases that widely presented in the cytoplasm and are able to cause phosphorylation of serine/threonine in the inner substrate protein molecule. The kinase property of MAPKs is mediated by the proline, i.e., only being able to phosphorylate the proline-containing substrate protein in P1 region. The major targeting substrate of MAPKs is transcription factor, also includes a number of protein kinases, phospholipases and cytoskeletal associated proteins. Dual phosphorylation of serine and tyrosine residues is a necessary condition for the activation of MAPKs. Different MKKs are able to recognize the spatial structure of the threonine X tyrosine domain in specific MAPKs, instead of just recognizing the linear sequence in the activation domain.
- Structure:
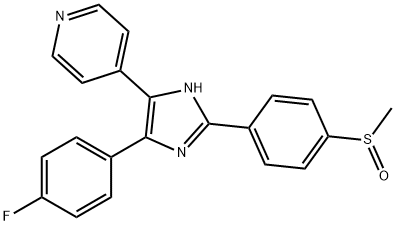
- Chemical Name:SB 203580
- CAS:152121-47-6
- MF:C21H16FN3OS
- Structure:
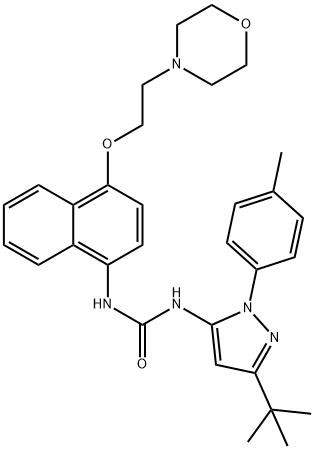
- Chemical Name:Doramapimod
- CAS:285983-48-4
- MF:C31H37N5O3
- Structure:
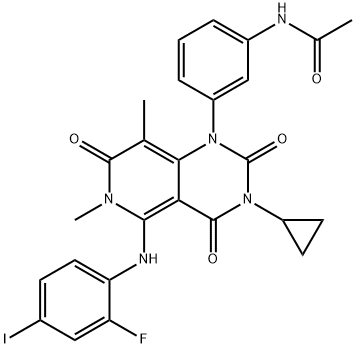
- Chemical Name:Trametinib
- CAS:871700-17-3
- MF:C26H23FIN5O4
- Structure:
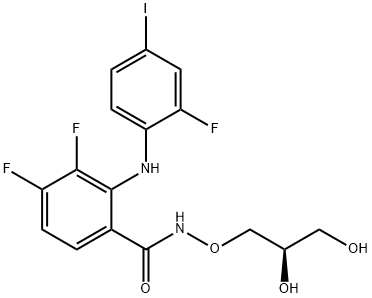
- Chemical Name:PD 0325901
- CAS:391210-10-9
- MF:C16H14F3IN2O4
- Structure:
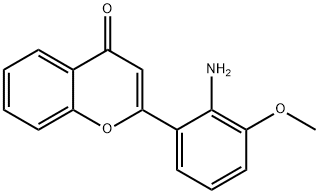
- Chemical Name:PD 98059
- CAS:167869-21-8
- MF:C16H13NO3
- Structure:
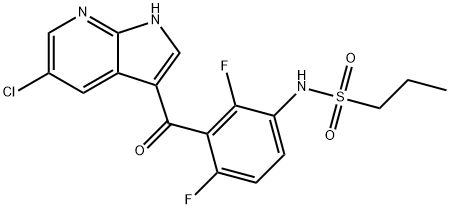
- Chemical Name:PLX-4720
- CAS:918505-84-7
- MF:C17H14ClF2N3O3S
- Structure:
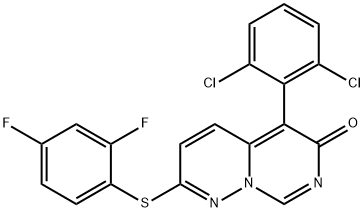
- Chemical Name:VX-745
- CAS:209410-46-8
- MF:C19H9Cl2F2N3OS
- Structure:
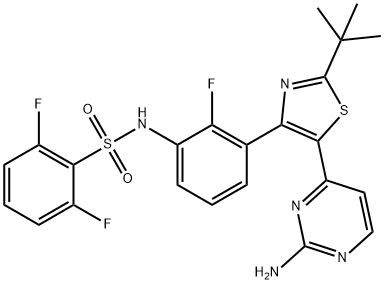
- Chemical Name:Dabrafenib
- CAS:1195765-45-7
- MF:C23H20F3N5O2S2
- Structure:
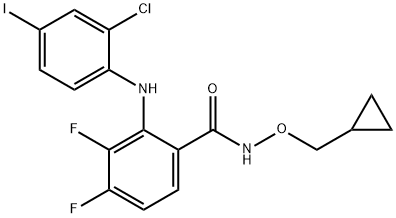
- Chemical Name:PD184352
- CAS:212631-79-3
- MF:C17H14ClF2IN2O2
- Structure:
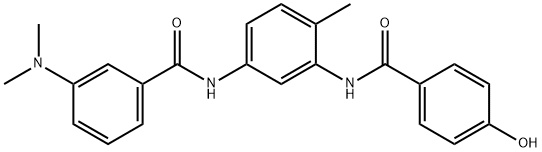
- Chemical Name:ZM 336372
- CAS:208260-29-1
- MF:C23H23N3O3
- Structure:
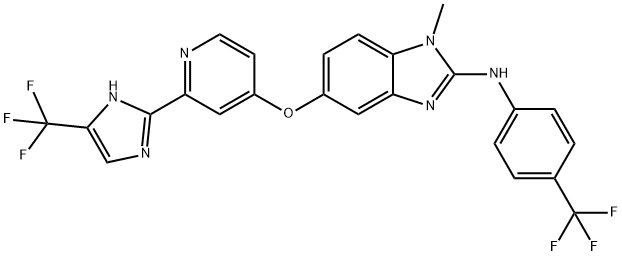
- Chemical Name:RAF265(CHIR-265)
- CAS:927880-90-8
- MF:C24H16F6N6O
- Structure:
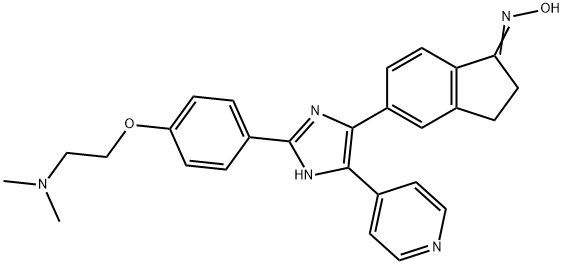
- Chemical Name:5-[2-[4-[2-(Dimethylamino)ethoxy]phenyl]-5-(4-pyridinyl)-1H-imidazol-4-yl]-2,3-dihydro-1H-inden-1-one oxime
- CAS:405554-55-4
- MF:C27H27N5O2
- Structure:
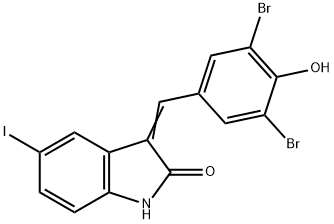
- Chemical Name:GW5074
- CAS:220904-83-6
- MF:C15H8Br2INO2
- Structure:
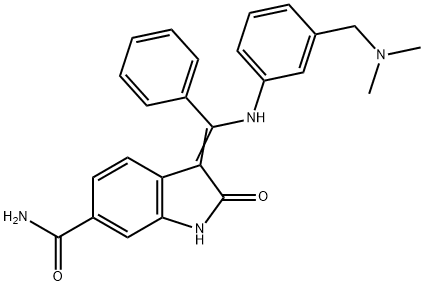
- Chemical Name:BIX 02188
- CAS:1094614-84-2
- MF:C25H24N4O2
- Structure:
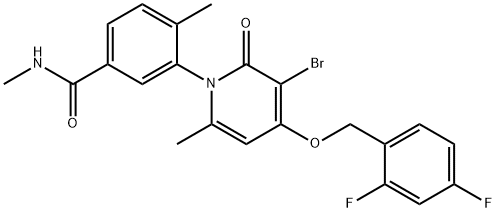
- Chemical Name:PH 797804
- CAS:586379-66-0
- MF:C22H19BrF2N2O3
- Structure:
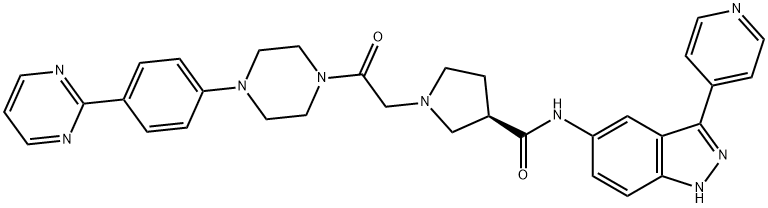
- Chemical Name:SCH772984
- CAS:942183-80-4
- MF:C33H33N9O2
- Structure:
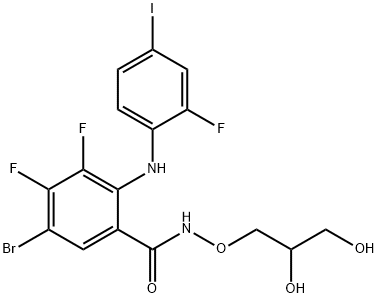
- Chemical Name:PD318088
- CAS:391210-00-7
- MF:C16H13BrF3IN2O4
- Structure:
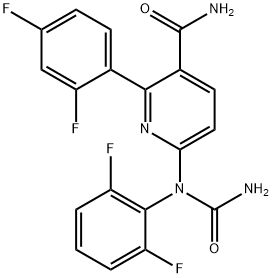
- Chemical Name:VX 702
- CAS:745833-23-2
- MF:C19H12F4N4O2
- Structure:
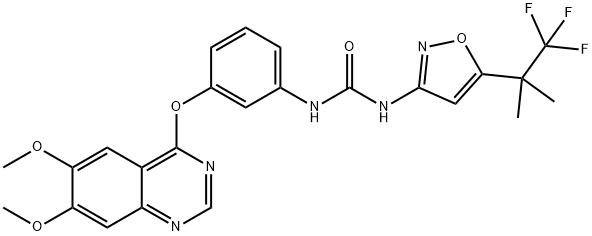
- Chemical Name:CEP-32496 (free base)
- CAS:1188910-76-0
- MF:C24H22F3N5O5
- Structure:
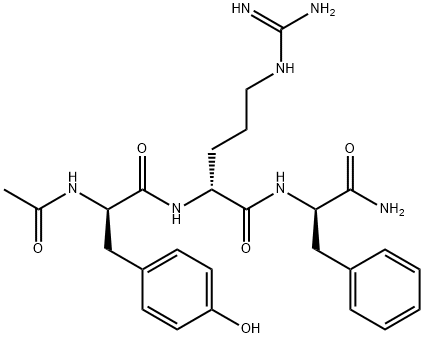
- Chemical Name:(R)-2-((R)-2-acetamido-3-(4-hydroxyphenyl)propanamido)-N-((R)-1-amino-1-oxo-3-phenylpropan-2-yl)-5-guanidinopentanamide
- CAS:1809784-29-9
- MF:C26H35N7O5
- Structure:
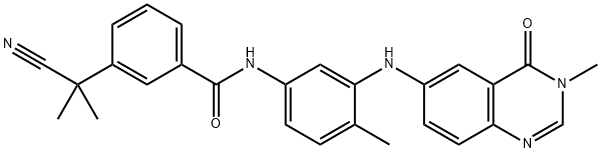
- Chemical Name:AZ 628
- CAS:878739-06-1
- MF:C27H25N5O2
- Structure:
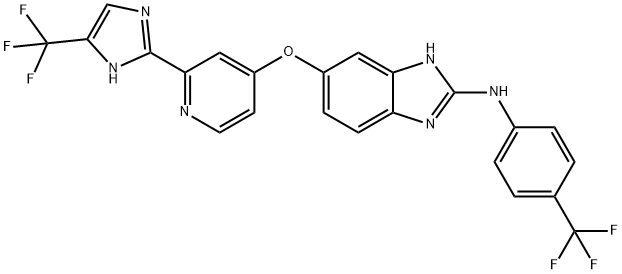
- Chemical Name:Raf265 derivative
- CAS:1942849-27-5
- MF:C23H14F6N6O
- Structure:
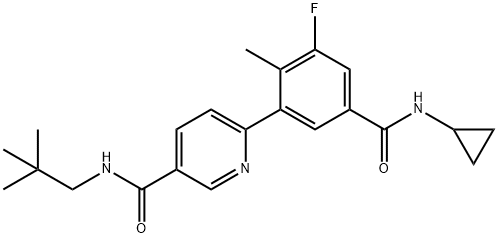
- Chemical Name:Losmapimod (GW856553X)
- CAS:585543-15-3
- MF:C22H26FN3O2
- Structure:
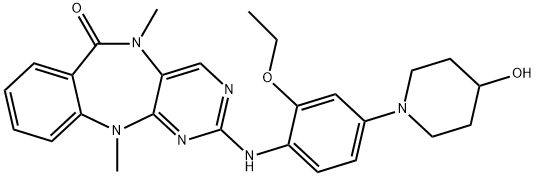
- Chemical Name:2-[[2-Ethoxy-4-(4-hydroxy-1-piperidinyl)phenyl]amino]-5,11-dihydro-5,11-dimethyl-6H-pyrimido[4,5-b][1,4]benzodiazepin-6-one
- CAS:1234480-50-2
- MF:C26H30N6O3
- Structure:
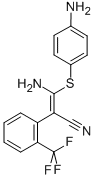
- Chemical Name:SL 327
- CAS:305350-87-2
- MF:C16H12F3N3S
- Structure:
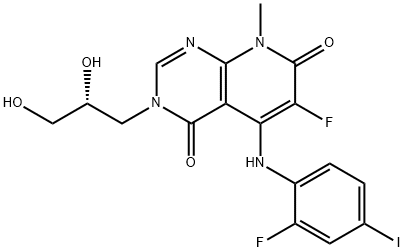
- Chemical Name:TAK-733
- CAS:1035555-63-5
- MF:C17H15F2IN4O4
- Structure:
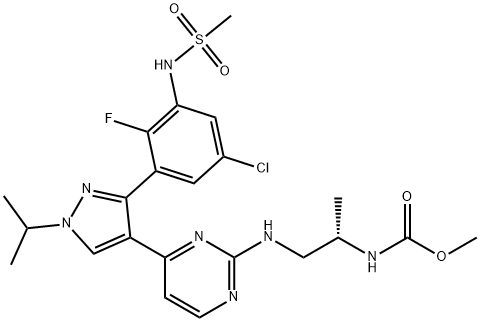
- Chemical Name:Encorafenib (LGX818)
- CAS:1269440-17-6
- MF:C22H27ClFN7O4S
- Structure:
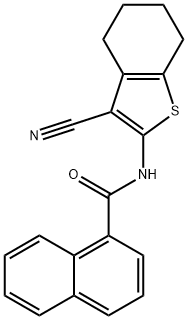
- Chemical Name:N-(3-Cyano-4,5,6,7-tetrahydrobenzo[b]thienyl-2-yl)-1-naphthalenecarboxamide
- CAS:312917-14-9
- MF:C20H16N2OS
- Structure:
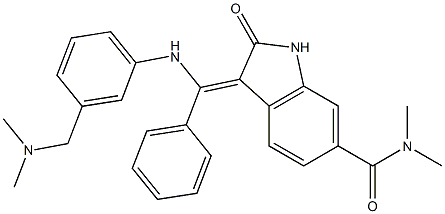
- Chemical Name:BIX 02189
- CAS:1094614-85-3
- MF:C27H28N4O2
- Structure:
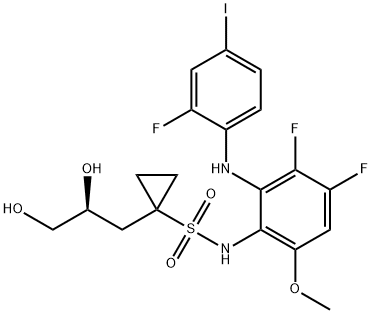
- Chemical Name:Refametinib
- CAS:923032-37-5
- MF:C19H20F3IN2O5S
- Structure:
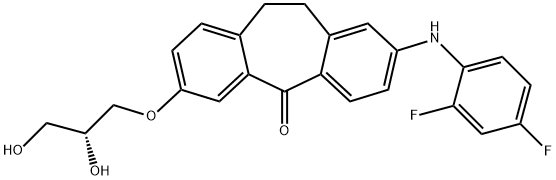
- Chemical Name:Skepinone-L
- CAS:1221485-83-1
- MF:C24H21F2NO4
- Structure:
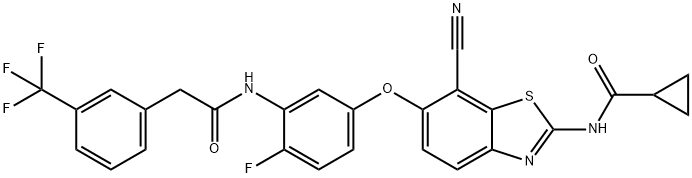
- Chemical Name:TAK-632
- CAS:1228591-30-7
- MF:C27H18F4N4O3S
- Structure:
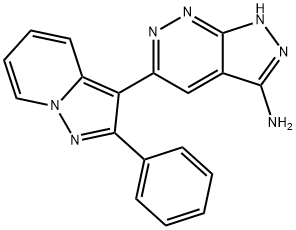
- Chemical Name:5-(2-Phenyl-pyrazolo[1,5-a]pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-ylamine
- CAS:865362-74-9
- MF:C18H13N7
- Structure:
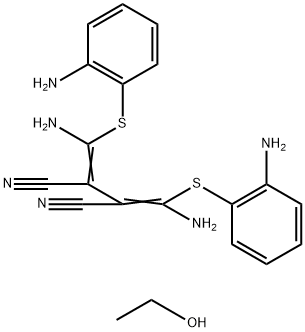
- Chemical Name:U0126-EtOH
- CAS:1173097-76-1
- MF:C20H22N6OS2
- Structure:
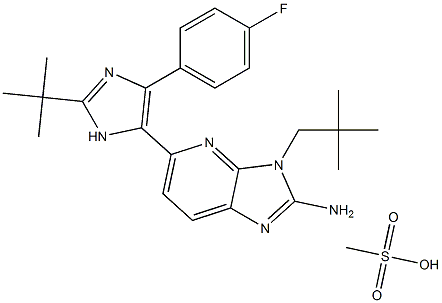
- Chemical Name:LY2228820
- CAS:862507-23-1
- MF:C26H37FN6O6S2
- Structure:
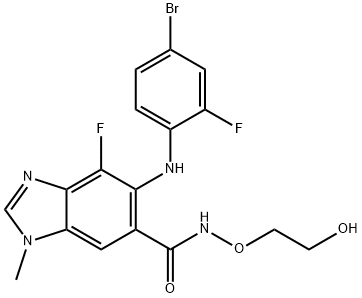
- Chemical Name:Binimetinib
- CAS:606143-89-9
- MF:C17H15BrF2N4O3
- Structure:
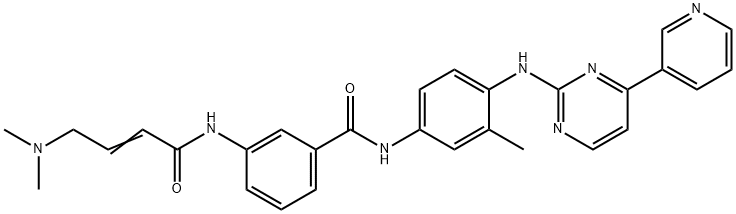
- Chemical Name:JNK-IN-8
- CAS:1410880-22-6
- MF:C29H29N7O2