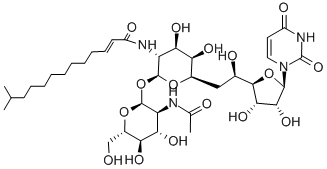
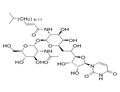
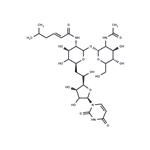
N-linked glycosylation is a highly regulated post-translational modification that is involved in protein folding and conformation, oligomerization, sorting, cell-cell interactions, and targeting of proteins to sub- or extra-cellular locations. It is initiated in the endoplastic reticulum with the transfer of a carbohydrate moiety to an asparagine residue within a specific amino acid consensus sequence and then further processed in the Golgi whereupon a mature glycoprotein is exported through the secretory machinery to the plasma membrane. Tunicamycin, a nucleoside antibiotic, is a specific inhibitor of N-linked glycoslylation that blocks the first step of glycoprotein synthesis thereby inducing protein unfolding. At 500 nM, it can impair the function of several receptor tyrosine kinases, including EGFR, ErbB2, ErbB3, and IGF-IR. Also at 500 nM, it radiosensitizes U251 glioma and BXPC3 pancreatic adenocarcinoma cells to chemotherapy. Tunicamycin impairs ALK phosphorylation and disrupts pro-survival signaling and cell viability in various neuroblastoma cell lines (IC50s range from 20 - 500 nM). Tunicamycin can also inhibit protein palmitoylation.