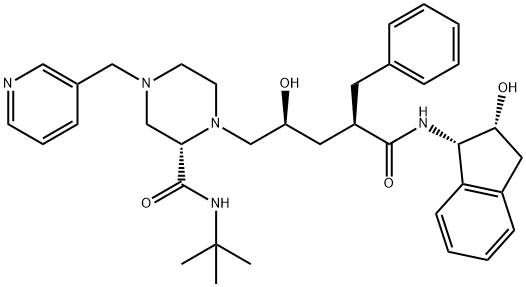
Crixivan was launched in Australia, Switzerland, the UK and the US
as an orally-bioavailable HIV-1 protease inhibitor. The compound can be prepared by
coupling an optically active piperazine derivative with an epoxide derivative (now
commercially available). The synthesis of the proteins, reverse transcriptase,
integrase, structural proteins and a protease, required by the virus to complete its
lifecycle, can be interupted if the protease enzyme is not capable of cleaving a proform
polypeptide chain into these components. lndinavir inhibits this process and is
more potent than the first approved protease inhibitor saquinavir. This effect was
noted by the increase in CD4+ cells and a decrease in HIV RNA levels. Since
indinavir is metabolized by the CYP3A4 isozyme, care must be taken with patients
with hepatic insufficiency and to sex-related differences in the level of this enzyme.
Other than nephrolithiasis (5%), indinavir is relatively safe and well tolerated.