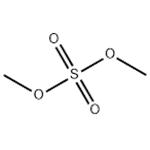
Dimethyl sulfate (chemical formula: (CH3O)2SO2) is an odorless, corrosive, oily liquid which can release toxic fumes during heating. It can be synthesized through the esterification of sulfuric acid with methanol, and alternatively by the distillation of methyl hydrogen sulfate. In industry, dimethyl sulfate is used as a methylating agent for the manufacture of many organic chemicals. It can be used for methylation of phenols, amines, and thiol. Moreover, it can be used for base sequencing and DNA chain cleavage since it can rupture the imidazole rings present in guanine. It can also be used for protein-DNA interaction analysis. However, its vapor is toxic to eyes and lungs, can do harm to our body. It is a potential carcinogen based on known experimental data.