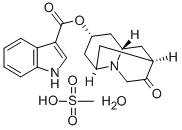
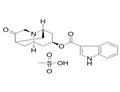
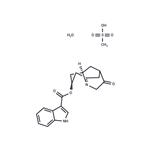
Dolasetron was launched as Anzemet in Australia and the US for the
prevention of nausea and vomiting in chemotherapy patients. It is a highly potent
and very selective antagonist of 5-HT3 receptors ; it is the sixth in this class of
compounds to be marketed for the treatment of chemotherapy-induced emesis.
The last two approved in this class were Nazasetron (1994) and Ramosetron
(1996). Anzemet was prepared by a seven step sequence from a
cyclopentenecarboxylic ester via a Robinson-Schopf cyclisation of a dialdehyde
into a key 9-azabicyclo[3.3.l]nonan-3-one. In a clinical study with 164 cancer
patients treated with Dolasetron mesylate prior to Cisplatin, single doses of 10-
50 mg achieved major control of nausea and emesis in 73% of subjects and
were well tolerated. Results from pharmacokinetic studies in humans showed
that the clinical effects and duration of action seem to be due mainly to a major
plasma metabolite rapidly formed and very potent itself, the (+) enantiomeric
alcohol obtained by enzymatic reduction of the cyclic ketone.