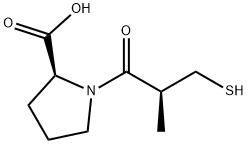
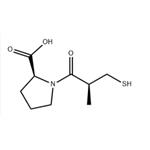
Angiotensin-converting enzyme (ACE) removes the C-terminal dipeptide from angiotensin I to form angiotensin II, a powerful vasoconstrictor. ACE is a key regulator of the renin-angiotensin system and an important drug target for the treatment of hypertension, congestive heart failure, and heart attacks, and also in preventing renal and retinal complications in diabetes. As the first nonpeptidic ACE inhibitor to be developed (IC50 = 6.3 nM), captopril was discovered based on bradykinin-potentiating peptides isolated from the venom of B. Jararaca, a pit viper native to Brazil. It does not exhibit a domain preference for binding either the C- or N-terminal active sites of the somatic form of ACE, which may account for some of captopril’s negative side effects. Captopril is also a competitive and reversible inhibitor of leukotriene (LTA4) hydrolase, which results in the disruption of LTB4 synthesis at an IC50 value of 14 μM.