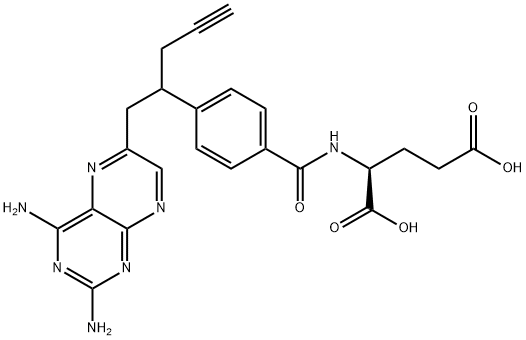
Pralatrexate, an injectable DHFR inhibitor, was launched for the treatment of patients with relapsed or refractory PTCL. PTCL is an
aggressive form of non-Hodgkin’s lymphoma (NHL) characterized by
the proliferation of abnormal T-lymphocytes that circulate in the peripheral bloodstream. The inhibition of the folate enzymes DHFR and thymidylate synthase is a well-validated method of cancer treatment.
In vitro, pralatrexate is
slightly less potent than MTX in inhibiting DHFR derived from murine
leukemia L1210 cells (Ki = 18.2 pM vs. 5.75 pM) and human leukemia
CCRF-CEM cells (Ki = 13.4 pM vs. 5.4 pM). However, it is transported
into both types of cells with 10-fold higher efficiency than MTX,
thereby providing a more potent inhibition of cell growth as compared
with MTX. In vivo, intraperitonally administered pralatrexate at 60 mg/
kg twice weekly for three or four doses caused complete lymphoma
regressions in 89, 56, and 30% of HT, RL, and SKI-DLBCL-1 xenografted
mice, respectively, whereas a similar dosing of MTX at 40 mg/kg twice
weekly did not produce complete regression. The posttreatment tumor
diameter was also smaller in pralatrexate-treated animals.