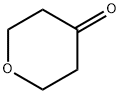
|
AK Scientific
E661
|
100g |
$107 |
Tetrahydro-4H-pyran-4-one |
|
|
Alichem
29943428
|
500g |
$237.44 |
Dihydro-2H-pyran-4(3H)-one |
|
|
Ambeed
A245086
|
1g |
$5 |
Dihydro-2H-pyran-4(3H)-one |
|
|
Ambeed
A245086
|
5g |
$6 |
Dihydro-2H-pyran-4(3H)-one |
|
|
Ambeed
A245086
|
10g |
$8 |
Dihydro-2H-pyran-4(3H)-one |
|
|
Ambeed
A245086
|
25g |
$16 |
Dihydro-2H-pyran-4(3H)-one |
|
|
American Custom Chemicals Corporation
CHM0032333
|
5G |
$779.63 |
TETRAHYDRO-4H-PYRAN-4-ONE |
|
|
American Custom Chemicals Corporation
CHM0032333
|
25G |
$1097.25 |
TETRAHYDRO-4H-PYRAN-4-ONE |
|
|
American Custom Chemicals Corporation
CHM0032333
|
100G |
$2517.9 |
TETRAHYDRO-4H-PYRAN-4-ONE |
|
|
Anichem
T14888
|
500g |
$650 |
TETRAHYDRO-4H-PYRAN-4-ONE |
|