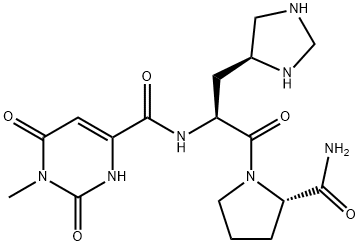
Taltirelin was marketed in Japan for the treatment of neurodegenerative diseases, in
particular the improvement of ataxia due to spino-cerebellar degeneration (SCD); it is the
first orally-active drug in this indication. This synthetic thyrotropin-releasing hormone (TRH)
analog is prepared by condensation of (S)-1-methyl-4,5-dihydroorotic acid with L-histidyl-Lprolinamide.
It was shown that the S-configuration for all 3 chiral centers is crucial for CNS activity. Taltirelin is a potent agonist of the TRH receptors that shows significant effects on
the cerebral monoamine systems when administered i.p. in rats. As TRH at 10-fold higher
doses, taltirelin has been found to increase the extracellular levels of dopamine and its
metabolites (DOPAC and HVA) as well as precursors and/or metabolites of noradrenaline
and serotonin in different areas of the rat brain. Taltirelin produced an anti-ataxic activity in
the Rolling mouse Nagoya, an ataxic mutant mouse. In several clinical studies performed
in patients suffering from various forms of SCD, taltirelin demonstrated a statistically
significant increase in the global improvement of ataxic symptoms and other neurologic abnormalities.