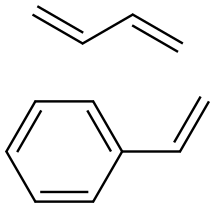
By far the most widely used type of synthetic rubber, its consumption for all applications is four times that of polybutadiene, its nearest competitor, and 1.5 times that of all other elastomers combined. Its manufacture involves copolymerization of three parts butadiene with one part styrene. These materials are suspended in finely divided emulsion form in a large proportion of water, in the presence of a soap or detergent. Also present in small amounts are an initiator or catalyst which is usually a peroxide, and a chain-modifying agent such as dodecyl mercaptan.