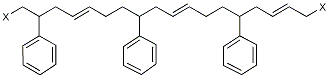
By far the most widely used type of synthetic rubber, its consumption for all applications is four times that of polybutadiene, its nearest competitor, and 1.5 times that of all other elastomers combined. Its manufacture involves copolymerization of three parts butadiene with one part styrene. These materials are suspended in finely divided emulsion form in a large proportion of water, in the presence of a soap or detergent. Also present in small amounts are an initiator or catalyst which is usually a peroxide, and a chain-modifying agent such as dodecyl mercaptan.
solid or viscous liquid, depending upon the degree of polymerisation
Component of hot melt adhesives, sealants, asphalt and oil gels. Modifier for polymers, thermosets and general rubber compounding. Direct molded or extruded auto parts, sporting goods, footware and film.
Molded or extruded into toys, packaging, appliances, communication cassettes, dinnerware and serviceware.
ChEBI: Styrene-butadiene copolymer is a polymer.
SBR is prepared mainly by emulsion and solution polymerization
in a free-radical process with a mercaptan, such as
dodecylmercaptan, which is used as a chain-transfer agent
to control molecular weight. In the emulsion process, a soapstabilized
water emulsion of styrene and butadiene is polymerized in continuous reactors to form SBR elastomer.
“Cold” SBR is produced at a temperature of 5–10°C, and
“hot” SBR at about 50°C . Solution SBR is typically
made in hydrocarbon solution with an n-butyllithium initiator.
The major differences between emulsion and solution
styrene–butadiene are in the linearity and molecular-weight
distribution of the polymer chains. In nonpolar solvents, the
tendency is to produce block copolymer, but random copolymers
are formed in polar solvents. SBR can be vulcanized
similar to natural rubber but generally requires less sulfur and
is more accelerated because of its lowunsaturation.
Styrene Butadiene Rubber is prepared in large quantities for tire treads and other, similar uses. The properties, molecular weight, and percentage of monomer can all be controlled in the polymerization process. Hazards of the SBR industry have been identified and related mostly to the monomers and additives. Contact dermatitis and leukemia have been associated with solvent and additive use but not with the actual polymer itself (Checkoway et al., 1984; Kilpikari, 1982). Epidemiological studies in the industry have not revealed a significant increase in disease or causes of death (Matanoski and Schwartz, 1987; Meinhardt et al., 1982). The hazards of the butadiene and styrene monomers were discussed previously.
An eye irritant. Questionable carcinogen. When heated to decomposition it emits acrid smoke and irritating fumes. See also POLYMERS, INSOLUBLE.
Chloroform, dichloromethane, hexane, MEK, toluene
Due to a lack of available toxicity data, the ITSL for Styrene-butadiene polymer is being set at 0.1 μg/m3 with annual averaging.