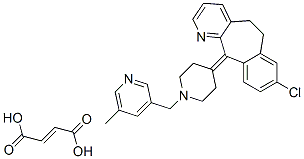
Rupatadine fumarate, a novel antiallergic drug with a dual mechanism of action, was
introduced in Spain as an oral treatment for perennial and seasonal rhinitis.
Rupatadine acts as non-sedating histamine H1 receptor antagonist and plateletactivating
factor (PAF) antagonist. Its Kiapp
i values against [3H]WEB-2086 binding to
rabbit platelet membrane PAF receptors and [3H]pyralimine binding to guinea pig
cerebellum membrane H1 histamine receptors are 0.55 and 0.10 μM, respectively. It
has a rapid onset of action, with patients experiencing relief of symptoms within 2 h,
and its long duration of action (>24 h) permits once-daily dosing. Rupatidine is
prepared in a 6-step convergent synthesis, with the key steps involving the Grignard
reaction of a N-alkyl-4-chloropiperdine with a benzocycloheptapyridinone intermediate,
followed by dehydration. Rupatadine is rapidly absorbed after oral administration.
The time to reach maximum plasma concentration is 0.75–1 h and the mean
half-life in healthy volunteers is ~6 h. It is extensively metabolized, mainly by
CYP3A4, and the major elimination route for the drug is biliary excretion. In
comparative clinical trials, rupatadine 10 mg once daily was as effective as certizine
10 mg in short-term studies (2–4 weeks duration), but provided a better profile of
CNS side effects. In comparison with ebastine 10 mg and loratadine 10 mg,
rupatadine showed a superior relief of rhinitis symptoms at the same dose.
Rupatadine was well tolerated in clinical trials and, at the recommended daily dose
of 10 mg, was free of the sedative effects associated with first-generation
antihistamines. In addition, there were no significant differences in the overall
incidence of adverse events in rupatidine-treated patients and those treated with
placebo or standard reference products.