Early explosives used nitric acid. They were so unstable to handle
that European scientists tried to find an explosive compound for
better safety. Around 1846, it was discovered that concentrated
nitric acid absorbed into cotton was not explosive until dried;
thus, guncotton was developed by chemical binding of nitrate
to cellulose. Preventing spontaneous explosions during the
manufacturing process required extensive washing and drying of
the cotton. In 1884, a French chemist made smokeless powders
from nitrocellulose. Their more stable and slower burning
properties enabled development of firearms and artillery
ammunitions. Eastman Kodak film products used nitrocellulose
as early as 1889. The film was used until 1933 for X-ray films and
for motion picture films until 1951.
Nitrocellulose can take on various physical forms, from
white fibers to thin sheets to thick liquid. Nitrocellulose can
also be a white, yellow, or transparent plastic. Its rigidity varies
from brittle to flexible. The unique properties enable nitrocellulose
to be used now in a wide variety of products. The variability
in physical properties comes from the content of
nitrogen and determines the use. The molecular weight of
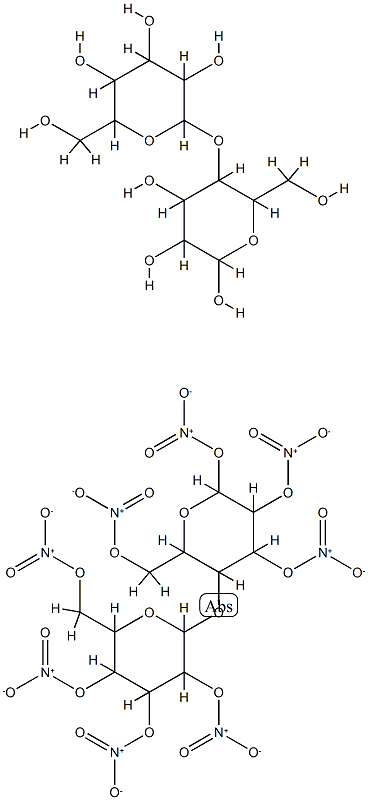
nitrocellulose ranges from 459.28 to 594.28, and the molecular
formula is expressed as [C6H7O2(ONO2)3]n. The hydroxyl
group of glucose units react to form nitrocellulose chains and
membranes.
Nitrocellulose is thus a fibrous solid polymer consisting of
the cellulose ester of nitric acid. Its specific gravity is 1.66. Its
form can be a white pulpy, cotton-like, amorphous solid in the
dried state, or a colorless liquid to semisolid, depending on the
degree of nitration. It has low water solubility; however, it is
soluble in 25% of a mixture of 1 volume of alcohol and 3
volumes of ether, forming collodion. Nitrocellulose is also
soluble in organic solvents such as methanol, acetone, glacial
acetic acid, and amyl acetate.