| Melting point |
1670°C |
| Density |
3.14 |
| refractive index |
1.63 |
| FEMA |
3081 | TRICALCIUM PHOSPHATE |
| storage temp. |
room temp |
| solubility |
Practically insoluble in water. It dissolves in dilute hydrochloric acid and in dilute nitric acid. |
| form |
aqueous suspension |
| color |
White |
| PH |
6-8 (50g/l, H2O, 20°C) suspension |
| Odor |
Odorless |
| Water Solubility |
0.1 g/L (25 ºC) |
| Merck |
13,1699 |
| Solubility Product Constant (Ksp) |
pKsp: 28.68 |
| Cosmetics Ingredients Functions |
ORAL CARE
ABRASIVE
ANTICAKING
FRAGRANCE
OPACIFYING |
| Cosmetic Ingredient Review (CIR) |
Calcium phosphate (7758-87-4) |
| InChI |
1S/3Ca.2H3O4P/c;;;2*1-5(2,3)4/h;;;2*(H3,1,2,3,4)/q3*+2;;/p-6 |
| InChIKey |
QORWJWZARLRLPR-UHFFFAOYSA-H |
| SMILES |
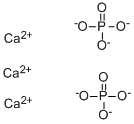
[Ca+2].[Ca+2].[Ca+2].[P](=O)([O-])([O-])[O-].[P](=O)([O-])([O-])[O-] |
| LogP |
-2.15 |
| CAS DataBase Reference |
7758-87-4(CAS DataBase Reference) |
| EPA Substance Registry System |
Tricalcium phosphate (7758-87-4) |