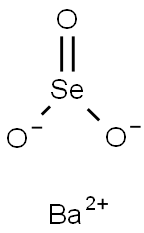
Barium selenite
$35.3 - $4396.22
- Product nameBarium selenite
- Cas No13718-59-7
- MFBaO3Se
- MW264.29
- EINECS237-280-0
- MDL NumberMFCD00003446
| Brand | Packaging | Price | Product name | |
|---|---|---|---|---|
|
American Custom Chemicals Corporation
ING0002455
|
100G | $2469.29 | BARIUM SELENITE |
|
|
American Custom Chemicals Corporation
ING0002455
|
500G | $4396.22 | BARIUM SELENITE |